Modified hydrophobic auxiliary material as well as preparation method and application thereof
A water auxiliary material and modification technology, applied in the field of modified hydrophobic auxiliary material and its preparation, can solve the problems of limited solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
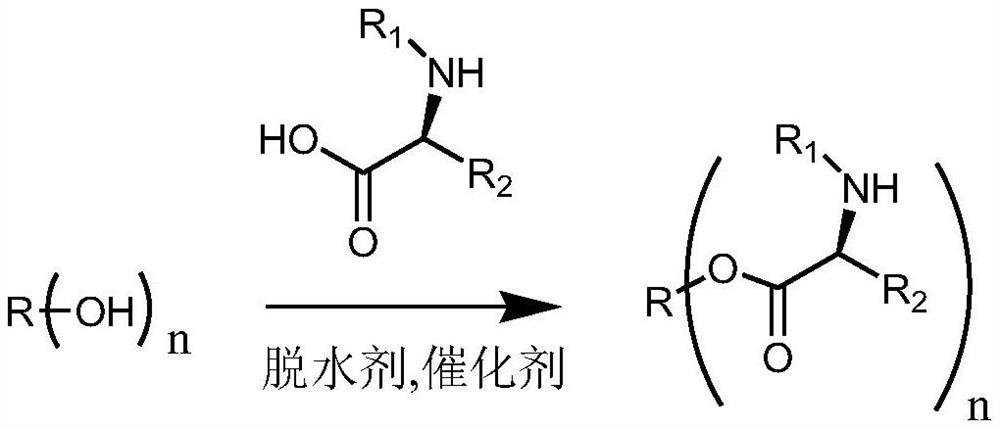
[0090] A preparation method of a modified hydrophobic auxiliary material, specifically as follows:
[0091] In a 250ml flask, add 9.34g castor oil (10mmol), 29.5gN-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-L-lysine (60mmol), 366mg4-dimethylaminopyridine (DMAP) ( 3mmol), then add dicyclohexylcarbodiimide (DCC) 12.4g (60mmol) dissolved in 50ml of anhydrous dichloromethane, at room temperature, react for three days under dark conditions, after TLC analysis has reacted completely , filtered to remove the precipitate and then spin-dried. After conventional purification, a slightly yellow transparent viscous oil was obtained. After cooling, it became a glassy transparent solid, and a modified hydrophobic auxiliary material was obtained. The reaction equation is shown below. Castor oil is usually a mixture of triglycerides with different ricinoleic acid content (n=0-3), and its molecular formula cannot be accurately represented. The following reaction equation is used to illus...
Embodiment 2
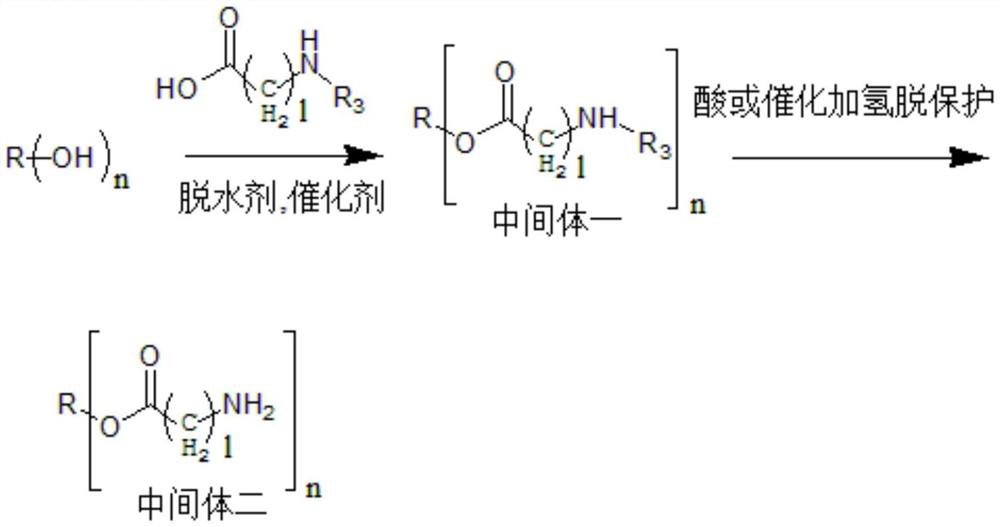
[0094] A preparation method of a hydrophobic auxiliary material, specifically as follows:
[0095] A-1, castor oil-O-glycine triester is esterified by N-tert-butoxycarbonylglycine, and then deprotects the synthetic route with acidification, specifically as follows:
[0096] In a 250ml flask, add 9.34g castor oil (10mmol), 7.2g N-tert-butoxycarbonylglycine (45mmol), 366mg 4-dimethylaminopyridine (DMAP) (3mmol), and then add 50ml of anhydrous dichloro N, N'-dicyclohexylcarbodiimide (DCC) 12.4g (60mmol) of methane was reacted at room temperature for two days in the dark, after the reaction was complete by TLC analysis, the precipitate was removed by filtration and spin-dried , a colorless transparent viscous oil was obtained after conventional purification, and Intermediate 1 was obtained, which was castor oil-O-(N-tert-butoxycarbonylglycine) triester.
[0097] Dissolve intermediate 1 in 40ml of dichloromethane (DCM), then add 40ml of trifluoroacetic acid (TFA), react at room te...
Embodiment 3
[0112] A preparation method of a hydrophobic auxiliary material, specifically as follows:
[0113] A, the synthesis of hydrogenated castor oil-O-glycine triester, specifically as follows:
[0114] In a 250ml flask, add 9.36g hydrogenated castor oil (10mmol), 7.2g N-tert-butoxycarbonylglycine (45mmol), 366mg4-dimethylaminopyridine (DMAP) (3mmol), dissolve in 100ml of anhydrous dichloromethane (DCM), then add 10.3 g (50 mmol) of dicyclohexylcarbodiimide (DCC) dissolved in 50 ml of anhydrous dichloromethane, reflux at a temperature of 45° C., and react for two days under light-shielding conditions. After the reaction was complete by TLC analysis, filter to remove the precipitate and spin dry. After recrystallization and purification from ethyl acetate, a slightly yellow transparent viscous oil was obtained. After cooling, it became white crystals to obtain Intermediate 1, which is hydrogenated castor oil. -O-(N-tert-butoxycarbonylglycine) triester.
[0115] Dissolve intermediat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com