Novel PEG (polyethylene glycol)-modified recombinant human granulocyte colony-stimulating factor preparation
A stimulating factor, polyethylene glycol technology, applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as blood pressure reduction, heart rate acceleration, hemolysis, etc., and achieve protein reduction. The formation of aggregates and the effect of enhancing the stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] In one embodiment, the preparation method of the pegylated recombinant human granulocyte-stimulating factor comprises the following steps:
[0025] (1) rhG-CSF reaction solution (40mL, concentration 3mg / mL), containing 100mM sodium phosphate, pH 5.0, also contains 120mM vitamin B6, after fully stirring and freezing (4°C), add 5 times the amount of protein Methoxypolyethylene glycol aldehyde (average molecular weight 20kDa). The reaction mixture was continuously stirred at the same temperature;
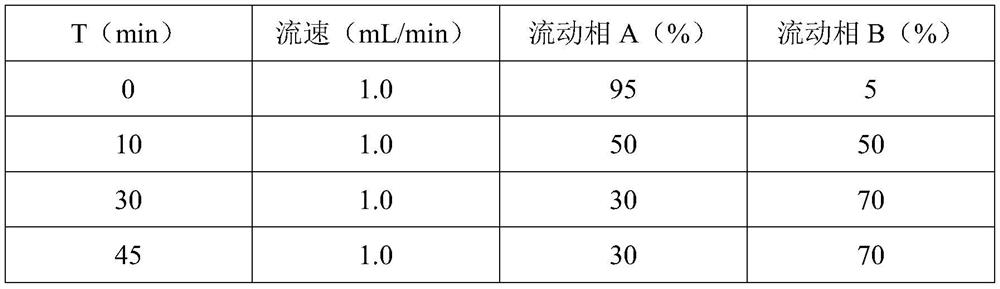
[0026] (2) The modification rate of protein in the reaction process is monitored by RP-HPLC. What RP-HPLC uses is C18 250×4.6mm 5 μm column (phenomenex), with 0.1% trifluoroacetic acid aqueous solution, 0.1% trifluoroacetic acid acetonitrile , gradient elution was performed at a flow rate of 1.0 mL / min.
[0027] In the present invention, the pegylated recombinant human granulocyte-stimulating factor is only suitable for non-myeloid cancer patients receiving myelosuppressive an...
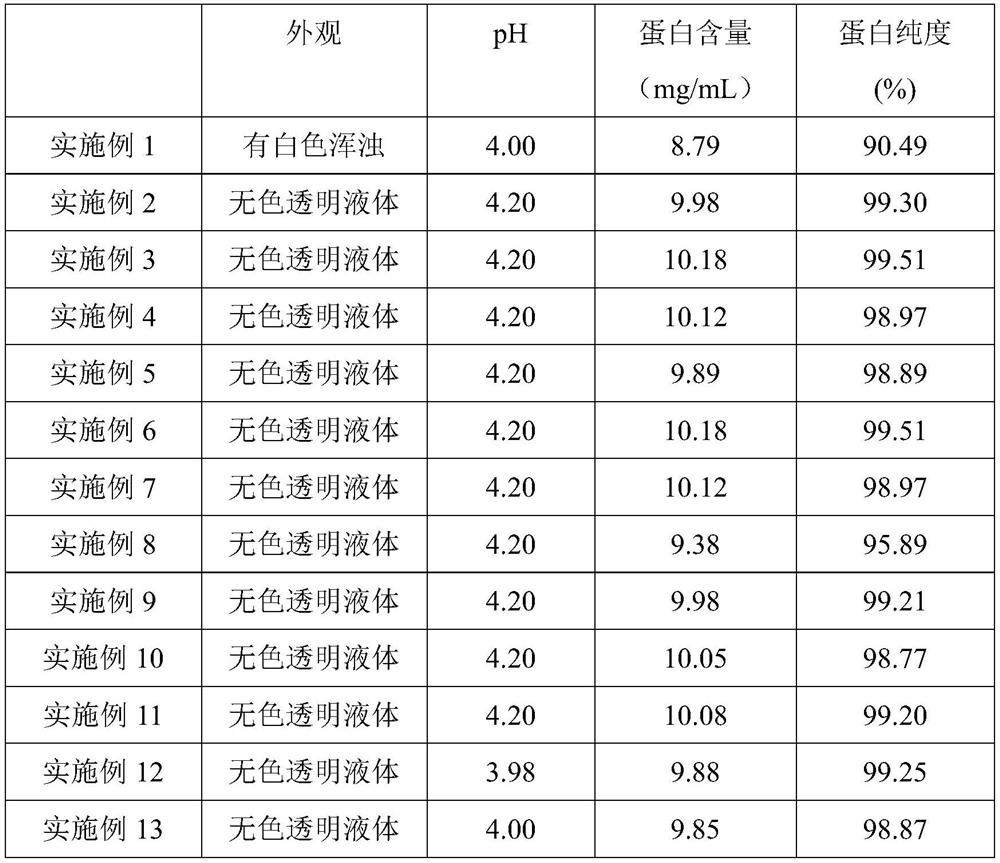
Embodiment 1
[0070] Example 1 provides a new preparation of polyethylene glycol-modified recombinant human granulocyte-stimulating factor, which consists of: PEGylated recombinant human granulocyte-stimulating factor 10 mg, citric acid 1.05 mg, sodium citrate 1.47 mg, D- Add 50mg of sorbitol, 0.87mg of arginine, and add water for injection to 1mL;
[0071] The preparation method of the PEGylated recombinant human granulocyte-stimulating factor comprises the following steps:
[0072](1) rhG-CSF reaction solution (40mL, concentration 3mg / mL), containing 100mM sodium phosphate, pH 5.0, also contains 120mM vitamin B6, after fully stirring and freezing (4°C), add 5 times the amount of protein Methoxypolyethylene glycol aldehyde (average molecular weight 20kDa). The reaction mixture was continuously stirred at the same temperature;
[0073] (2) The modification rate of protein in the reaction process is monitored by RP-HPLC. What RP-HPLC uses is C18 250×4.6mm 5 μm column (phenomenex), with 0.1...
Embodiment 2
[0076] Example 2 provides a new preparation of polyethylene glycol-modified recombinant human granulocyte-stimulating factor, which consists of: PEGylated recombinant human granulocyte-stimulating factor 10 mg, citric acid 2.1 mg, sodium citrate 2.94 mg, D- Add 50mg of sorbitol, 0.87mg of arginine, and add water for injection to 1mL;
[0077] The preparation method of the PEGylated recombinant human granulocyte-stimulating factor is the same as that in Example 1.
[0078] The preparation method of the new preparation of polyethylene glycol-modified recombinant human granulocyte-stimulating factor is the same as that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com