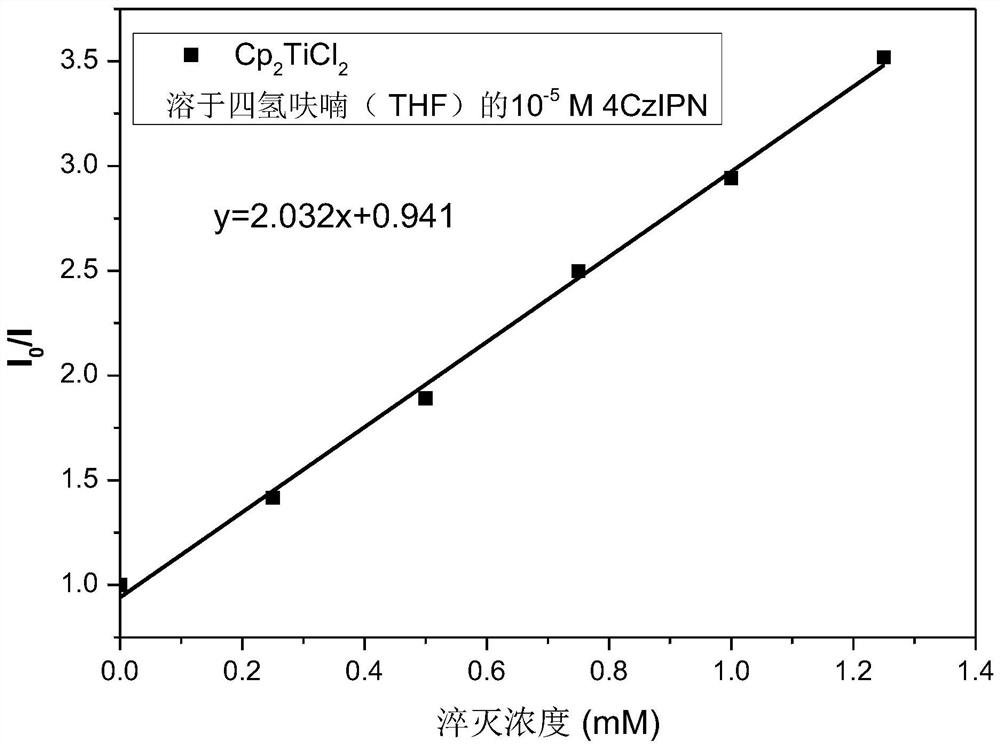
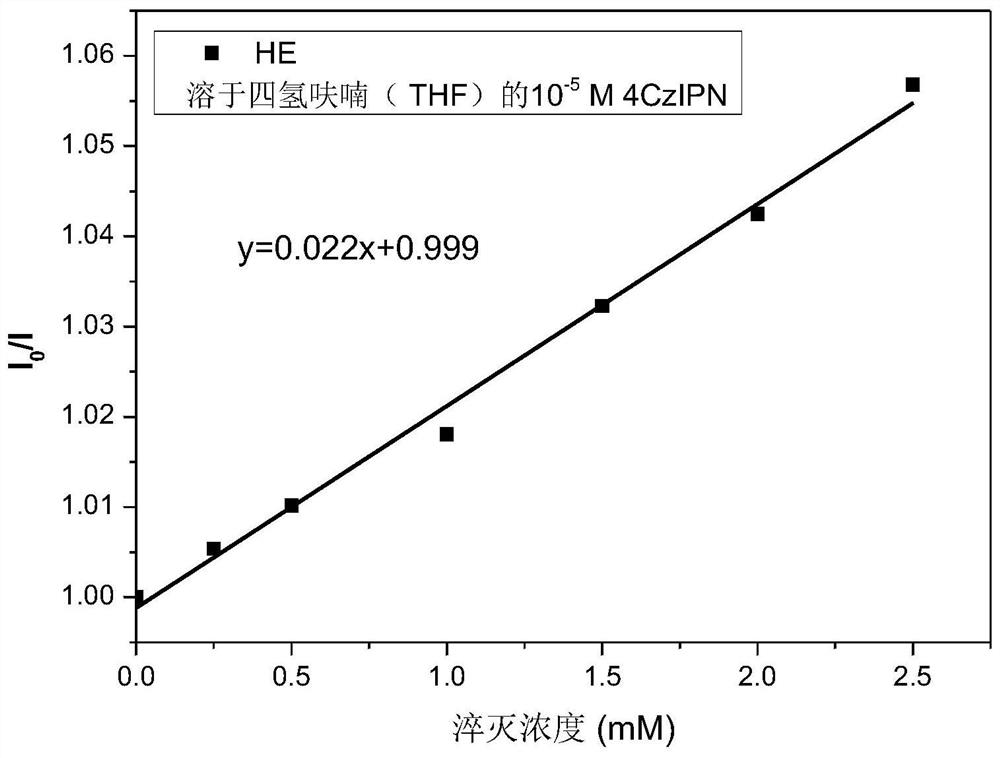
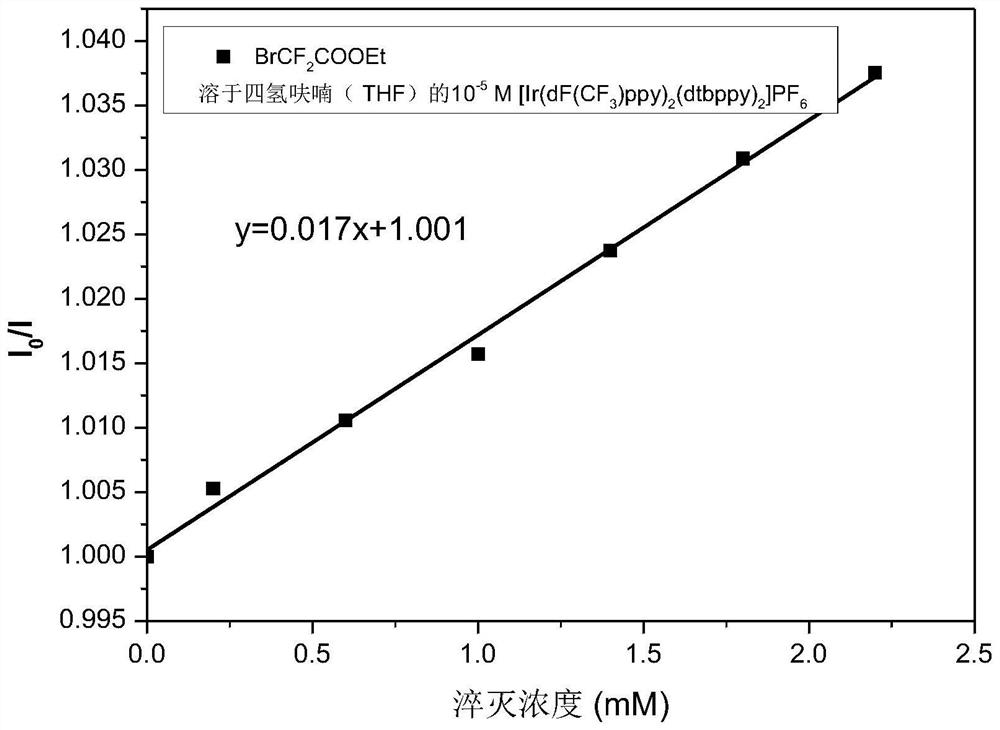
Preparation of homoallylic alcohol compound and synthesis method and application thereof
A high allyl alcohol, synthesis method technology, applied in the direction of steroids, organic chemical methods, chemical instruments and methods, etc., can solve the problems of uneconomical and cumbersome methods, and achieve high selectivity, high reaction yield, and reaction The effect of short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] The experimental steps and purification methods were carried out according to the exploratory experiments. RF=0.4 (EA:PE=1:3), eluent: petroleum ether / ethyl acetate=20 / 1, the yield was 92%, and the product was a colorless liquid. 1 H NMR (400MHz, CDCl3) δ7.35–7.27(m,2H),7.24–7.17(m,3H),5.71(dt,J=17.2,9.8Hz,1H),5.23–5.08(m,2H), 4.28(q, J=7.1Hz, 2H), 3.62(dt, J=8.3, 4.2Hz, 1H), 2.88–2.58(m, 2H), 2.51–2.41(m, 1H), 2.40–2.24(m, 2H), 2.01(s, 1H), 1.84–1.65(m, 2H), 1.34(t, J=7.2Hz, 3H). 13 C NMR (101MHz, CDCl3) δ164.08(t, J=33.0Hz), 141.62, 135.77, 128.35, 128.29, 125.84, 118.55, 116.57(dd, J=249.2, 248.7Hz), 72.91, 62.67, 43.59(dd , J=4.3, 2.3Hz), 36.24, 35.89 (t, J=22.7Hz), 32.05, 13.73. 19 F NMR (377MHz, CDCl3) δ-101.44 (dt, J = 261.5, 15.0Hz, 1F), -105.06 (dt, J = 260.7, 17.9Hz, 1F). HRMS-ESI (m / z) [M+Na ] + calculated for C 17 h 22 f 2 NaO 3 ,335.1435,found 335.1431.
Embodiment 2
[0035] use replace The experimental steps and purification methods were carried out according to the exploratory experiments. RF=0.4 (EA:PE=1:3), eluent: petroleum ether / ethyl acetate=20 / 1, the yield was 71%, and the product was a colorless liquid. 1 H NMR (400MHz, CDCl3) δ5.75 (dt, J = 18.3, 9.3Hz, 1H), 5.24–4.97 (m, 2H), 4.26 (q, J = 7.1Hz, 2H), 2.76 (dd, J = 9.0,4.2Hz,1H),2.53(dq,J=9.1,4.6Hz,1H),2.47–2.16(m,2H),1.87(s,1H),1.32(t,J=7.1Hz,3H), 1.01–0.83(m,1H),0.68–0.40(m,2H),0.37–0.10(m,2H). 13 C NMR (101MHz, CDCl3) δ164.13(t, J=32.7Hz), 136.55, 118.19, 116.15(dd, J=251.7, 248.9Hz), 78.85, 62.64, 44.22(dd, J=4.9, 2.5Hz) ,35.87(t,J=22.8Hz),15.17,13.81,3.12,2.73. 19 F NMR (377MHz, CDCl3) δ-101.43 (dt, J = 261.5, 15.0Hz, 1F), -105.04 (dt, J = 260.7, 17.9Hz, 1F). HRMS-ESI (m / z) [M+Na ] + calculated for C 12 h 18 f 2 NaO 3 ,271.1122,found 271.1137.
Embodiment 3
[0037] use replace The experimental steps and purification methods were carried out according to the exploratory experiments. RF=0.4 (EA:PE=1:3), eluent: petroleum ether / ethyl acetate=20 / 1, the yield was 74%, and the product was a colorless liquid. 1H NMR (400MHz, CDCl3) δ5.77–5.51(m,1H),5.22–5.12(m,3H),4.27(q,J=7.1Hz,2H),4.21(dd,J=9.0,6.5Hz, 1H), 2.38(ddt, J=12.9, 6.2, 2.9Hz, 1H), 2.34–2.22(m, 1H), 2.21–2.07(m, 1H), 1.76(d, J=1.4Hz, 3H), 1.73 (dd,J=3.3,1.4Hz,1H),1.69(d,J=1.4Hz,3H),1.34(t,J=7.2Hz,3H). 13 C NMR (101MHz, CDCl3) δ164.10 (t, J = 32.8Hz), 137.74, 136.95, 124.51, 119.11, 116.06 (dd, J = 252.0, 249.0Hz), 69.95, 62.70, 44.94 (dd, J = 4.7 ,2.7Hz), 35.33(t, J=22.9Hz), 25.93, 18.48, 13.89. 19 F NMR (377MHz, CDCl3) δ-100.34(t, J=14.8Hz), -101.04(t, J=14.6Hz), -104.02(t, J=18.0Hz), -104.71(t, J=17.8Hz ).HRMS-ESI(m / z)[M+Na] + calculated for C 13 h 20 f 2 NaO 3 ,285.1278,found285.1268.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com