Synthesis method of 3-isochromanone
A synthesis method and technology of isochromone, applied in the direction of organic chemistry, etc., can solve the problems of restricting industrialized large-scale production, long reaction time, low conversion rate, etc. The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The synthetic method of 3-isochromone of the present invention comprises the steps:
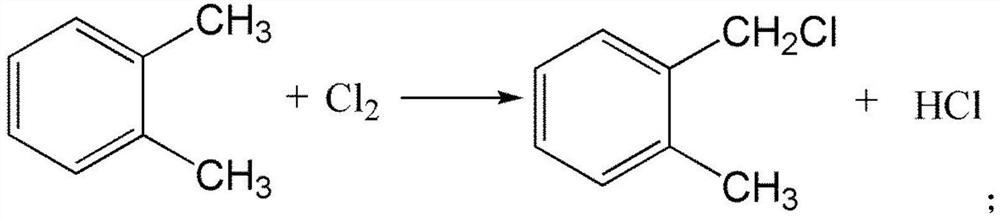
[0033] (1) take o-xylene as raw material to synthesize o-methyl benzyl chloride, and the reaction equation is as follows:
[0034]
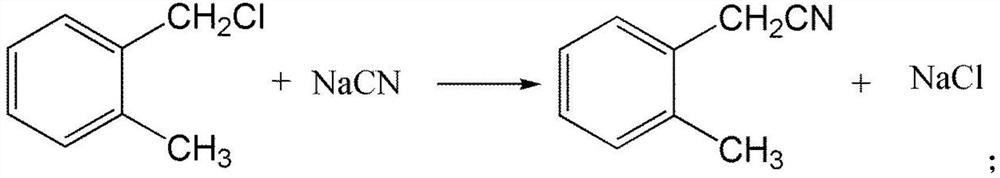
[0035] (2) take o-methyl benzyl chloride as raw material to synthesize o-toluene acetonitrile, and the reaction equation is as follows:
[0036]
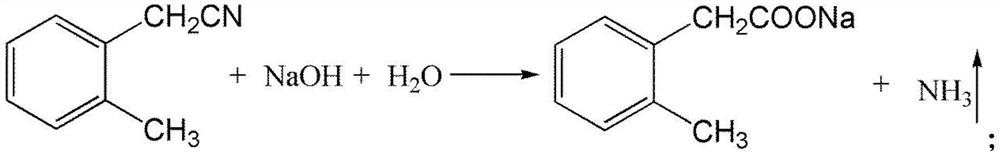
[0037] (3) take o-toluene acetonitrile as raw material to synthesize sodium o-toluene acetate, reaction equation is as follows:
[0038]
[0039] (4) take sodium o-toluene acetate as raw material to synthesize o-toluene acetic acid, and the reaction equation is as follows:
[0040]
[0041] (5) take o-methylphenylacetic acid as raw material synthetic 2-chloromethylphenylacetic acid, reaction equation is as follows:
[0042]
[0043] (6) take 2-chloromethylphenylacetic acid as raw material synthetic 3-isochromone, and reaction equation is as follows:
[0044]
Embodiment 1
[0046] The synthetic method of 3-isochromone described in the present embodiment comprises the steps:
[0047] (1) take o-xylene as raw material to synthesize o-methyl benzyl chloride;
[0048] The concrete process of step (1) is in 3000 liters of reaction flasks, first adds 2332g (22mol) o-xylene and heats up to 100 ℃, then passes into 781g (11mol) chlorine gas, reacts 5h under the effect of light source, the wavelength of light source Select at 2500nm to obtain o-methylbenzyl chloride, and then carry out rectification and purification on the obtained o-methylbenzyl chloride to obtain o-methylbenzyl chloride with a purity of more than 99%.
[0049] (2) take o-methyl benzyl chloride as raw material to synthesize o-toluene acetonitrile;
[0050] The concrete process of step (2) is in 3000 liters of reaction bottles, add the aqueous solution 1960g (wherein sodium cyanide is 12mol) of the sodium cyanide of 30% by mass percentage, be warming up to 100 ℃, dropwise add o-methyl ben...
Embodiment 2
[0060] The synthetic method of 3-isochromone described in the present embodiment comprises the steps:
[0061] (1) take o-xylene as raw material to synthesize o-methyl benzyl chloride;
[0062] The specific process of step (1) is to add 2332g (22mol) o-xylene to 95°C at first in a 3000-liter reaction bottle, then feed 937.2g (13.2mol) chlorine gas, and react for 4 hours under the action of a light source. The wavelength is selected at 3000nm to obtain o-methylbenzyl chloride, and then the obtained o-methylbenzyl chloride is subjected to rectification and purification to obtain o-methylbenzyl chloride with a purity of more than 99%.
[0063] (2) take o-methyl benzyl chloride as raw material to synthesize o-toluene acetonitrile;
[0064] The concrete process of step (2) is in 3000 liters of reaction bottles, add the aqueous solution 2352g (wherein sodium cyanide is 12mol) of the sodium cyanide of 25% by mass percent, be warming up to 30 ℃, dropwise add o-methyl benzyl chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com