Trimetal-based Ni-Co-Zn-N co-doped porous carbon catalyst as well as preparation method and application thereof
A technology of co-doping and porous carbon, applied in electrical components, battery electrodes, circuits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
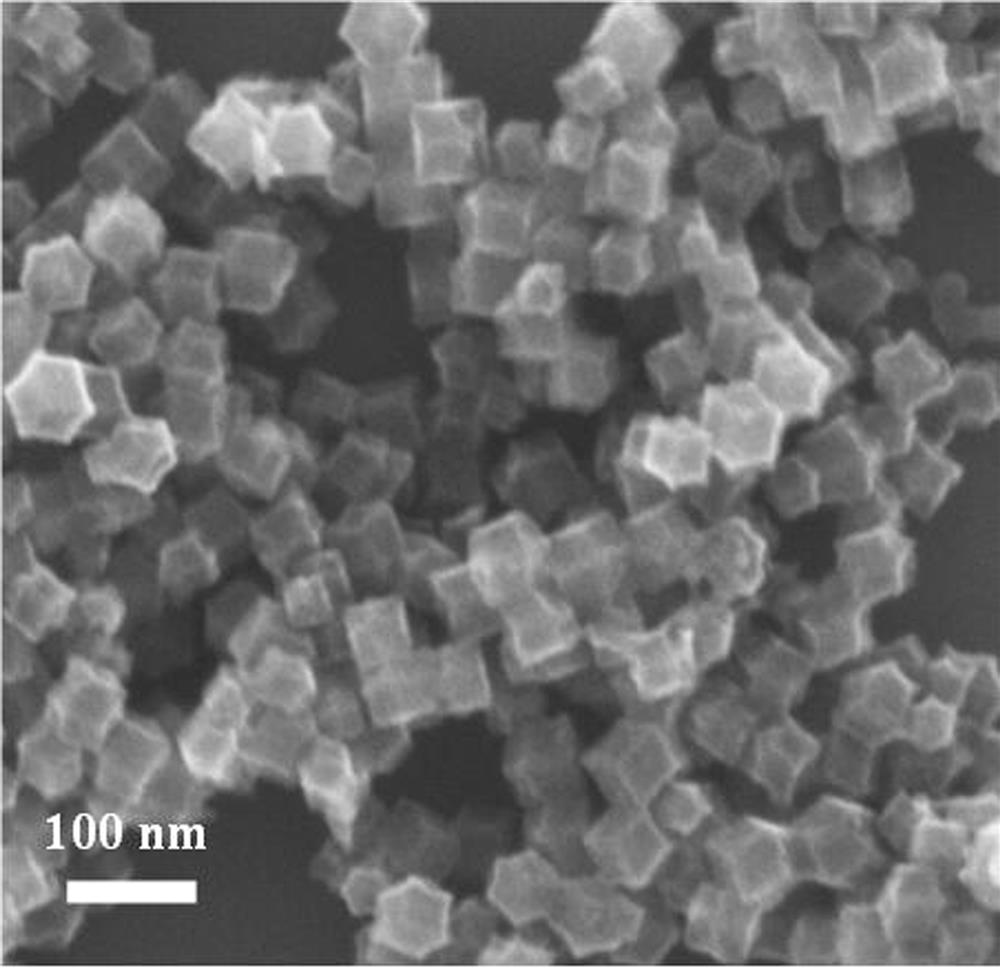
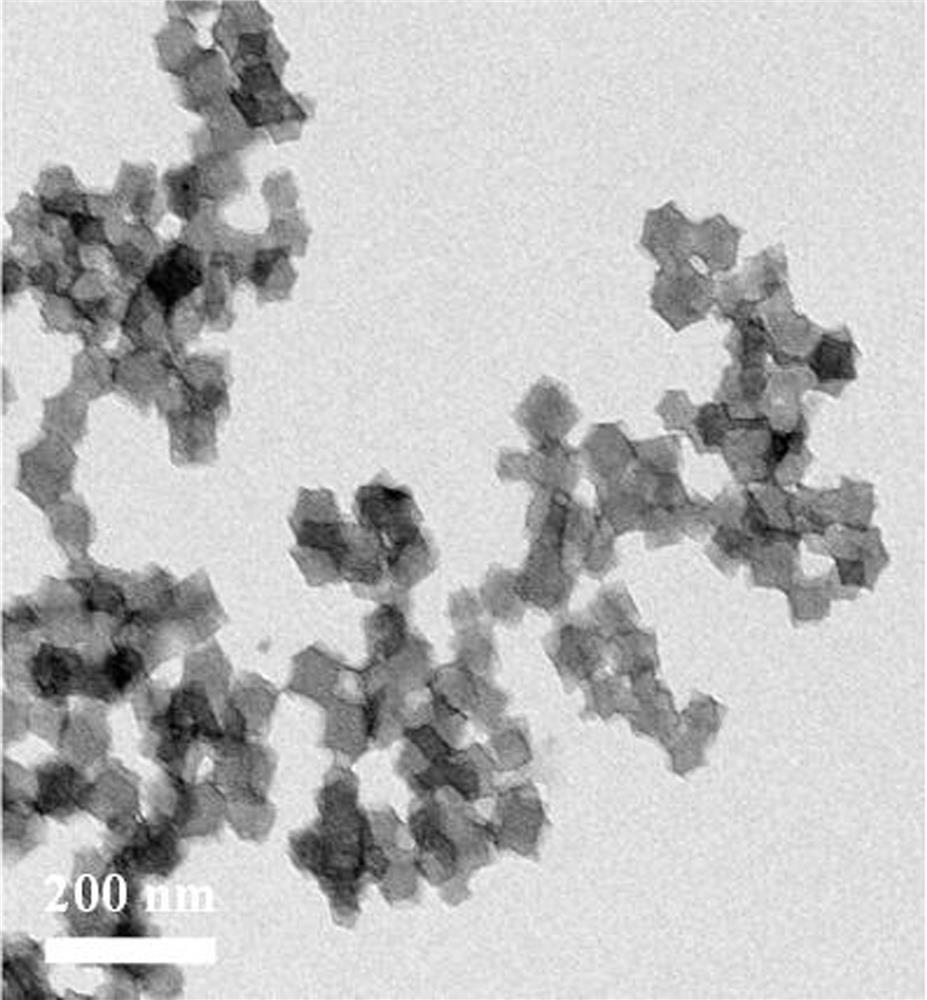
[0022] refer to figure 1 , a kind of preparation method of trimetallic base Ni-Co-Zn-N co-doped porous carbon catalyst, comprises the steps:
[0023]1) Add 5-30 mmol 2-methylimidazole to a container containing 35 mL of methanol, ultrasonically disperse evenly and add to 35 mL containing 0.05-1 mmol nickel nitrate, 0.05-1 mmol cobalt nitrate and 1-10 mmol zinc nitrate In the methanol solution, continue to sonicate for 10 minutes and then magnetically stir for 12 hours, centrifuge, wash, and dry to obtain a light purple nickel-cobalt doped zinc-based zeolite imidazole framework, that is, the Ni-Co-ZIF-8 precursor;
[0024] 2) Place the obtained precursor Ni-Co-ZIF-8 sample in a quartz tube furnace under N 2 The Ni-Co-Zn-N co-doped porous carbon catalyst, namely Ni-Co-Zn-N-PC, can be prepared by thermally decomposing at 600-1000° C. for 1-3 hours under protection.
[0025] Ni-Co-Zn-N co-doped porous carbon catalyst Ni-Co-Zn-N-PC prepared by the above preparation method.
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com