Catalyst in-situ preparation device for flow battery and catalyst preparation method
A flow battery, in-situ preparation technology, applied in fuel cells, battery electrodes, electrochemical generators, etc., can solve problems such as scarcity of research, achieve uniform distribution, reduce flow resistance, and improve uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
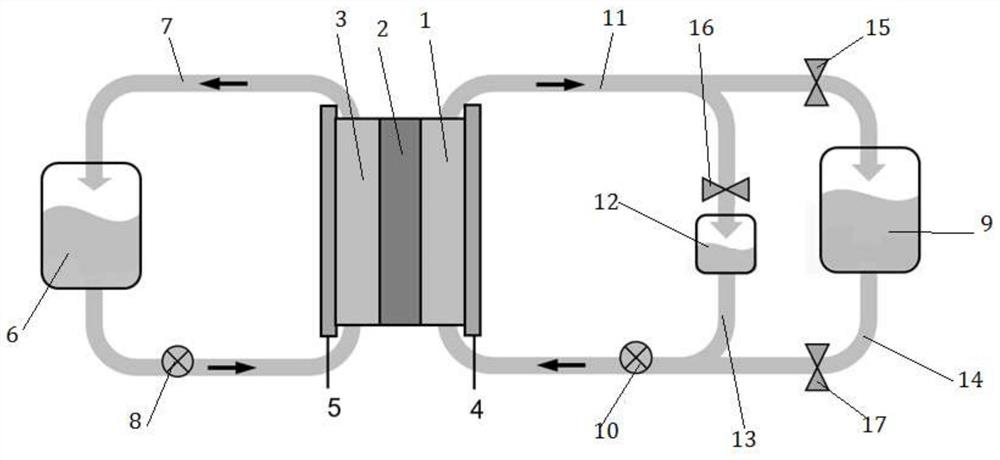
[0027] Such as figure 1 As shown, the single cell area of the all-vanadium redox flow battery is 30x 50cm 2150L of electrolyte is stored in the positive and negative liquid storage tanks, and the vanadium ion concentration is 1.5M / L. The storage tank of the bypass plating solution is only 1.0L, and the concentration of bismuth chloride is 8mM / L. During electroplating, valve 16 is opened, valves 15 and 17 are closed, and the negative electrode of the vanadium battery is switched to the electroplating bypass. During electroplating, the flow rate is 3L / min, and the electroplating current density used is 10mA cm -2 , the electroplating process works for 1 second, rests for 2 seconds, and uses this as a cycle for 60 cycles, and then uses a constant current of 3mA cm -2 Electroplating was performed to direct the complete consumption of bismuth ions. After the electroplating is completed, close the valve 16, open the valves 15 and 17, and the battery is charged and discharged n...
Embodiment 2
[0029] Such as figure 1 As shown, the all-vanadium redox flow battery stack, 3 layers of single cells are connected in series, and the area of each single cell is 30x50cm 2 , the positive and negative liquid storage tanks each store 250L electrolyte, in which the concentration of vanadium ions is 1.5M / L. The bypass electroplating solution storage tank is only 1.5L, and the concentration of bismuth chloride is 14mM / L. During electroplating, valve 16 is opened, valves 15 and 17 are closed, and the negative electrode of the vanadium battery is switched to the electroplating bypass. During electroplating, the flow rate is 8L / min, and the electroplating current density used is 8mA cm -2 , the electroplating process works for 0.5 seconds, rests for 1 second, and uses this as a cycle for 100 cycles, and then conducts electroplating with a constant current of 2mA cm-2 to guide the complete consumption of bismuth ions. After the electroplating is completed, close the valve 16, ope...
Embodiment 3
[0031] Such as figure 1 As shown, the all-vanadium redox flow battery stack, 3 layers of single cells are connected in series, and the area of each single cell is 30x50cm 2 , the positive and negative liquid storage tanks each store 250L electrolyte, in which the concentration of vanadium ions is 1.5M / L. The bypass electroplating solution storage tank is only 1.5L, and the concentration of bismuth chloride is 2mM / L. During electroplating, valve 16 is opened, valves 15 and 17 are closed, and the negative electrode of the vanadium battery is switched to the electroplating bypass. During electroplating, the flow rate is 8L / min, and the electroplating current density used is 5mA cm -2 , the electroplating process works for 0.1 second, rests for 1 second, and uses this as a cycle for 100 cycles, and then conducts electroplating with a constant current of 2mA cm-2 to guide the complete consumption of bismuth ions. After the electroplating is completed, close the valve 16, open ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com