Preparation method of betamethasone rearrangement substance and betamethasone rearrangement substance
A betamethasone and rearrangement technology, applied in the chemical industry, can solve the problems of large environmental pollution, low yield, high process cost, etc., and achieve the effects of low preparation cost, high yield, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
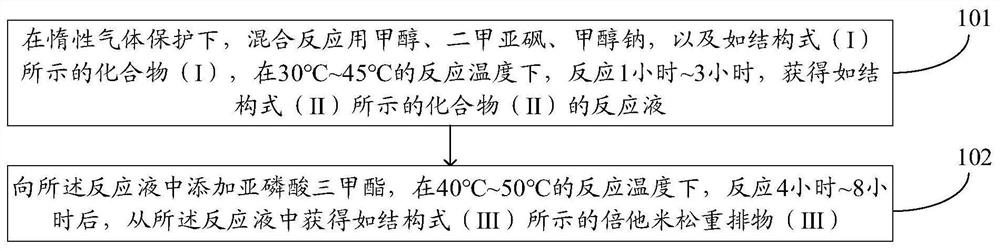
[0037] figure 1 It is a flow chart of the steps of a method for preparing a betamethasone rearrangement product provided by an embodiment of the present invention, and the method may include:
[0038] Step 101. Under the protection of an inert gas, use methanol, dimethyl sulfoxide, sodium methoxide, and compound (I) as shown in the structural formula (I) for the mixed reaction at a reaction temperature of 30°C to 45°C for 1 hour After ~3 hours, the reaction solution of compound (II) represented by structural formula (II) was obtained.
[0039] In the embodiment of the present invention, the compound (I) is 21-(phenylsulfinyl)pregna-16β-methyl-1,4,9(11)17(20), 20 as shown in the structural formula (I) - Pentaen-3-one, mixing compound (I) with methanol, dimethylsulfoxide (DMSO) and sodium methoxide for reaction, thereby performing double bond addition reaction on compound (I) to obtain compound (II), DMSO in the system significantly improves the nucleophilicity of sodium metho...
Embodiment 1
[0067] Under the protection of an inert gas, 100g of compound (I), 200mL of methanol, 300mL of DMSO, and 25.1g of sodium methoxide were uniformly mixed, and reacted at a reaction temperature of 30°C for 2 hours to obtain a reaction solution containing compound (II);
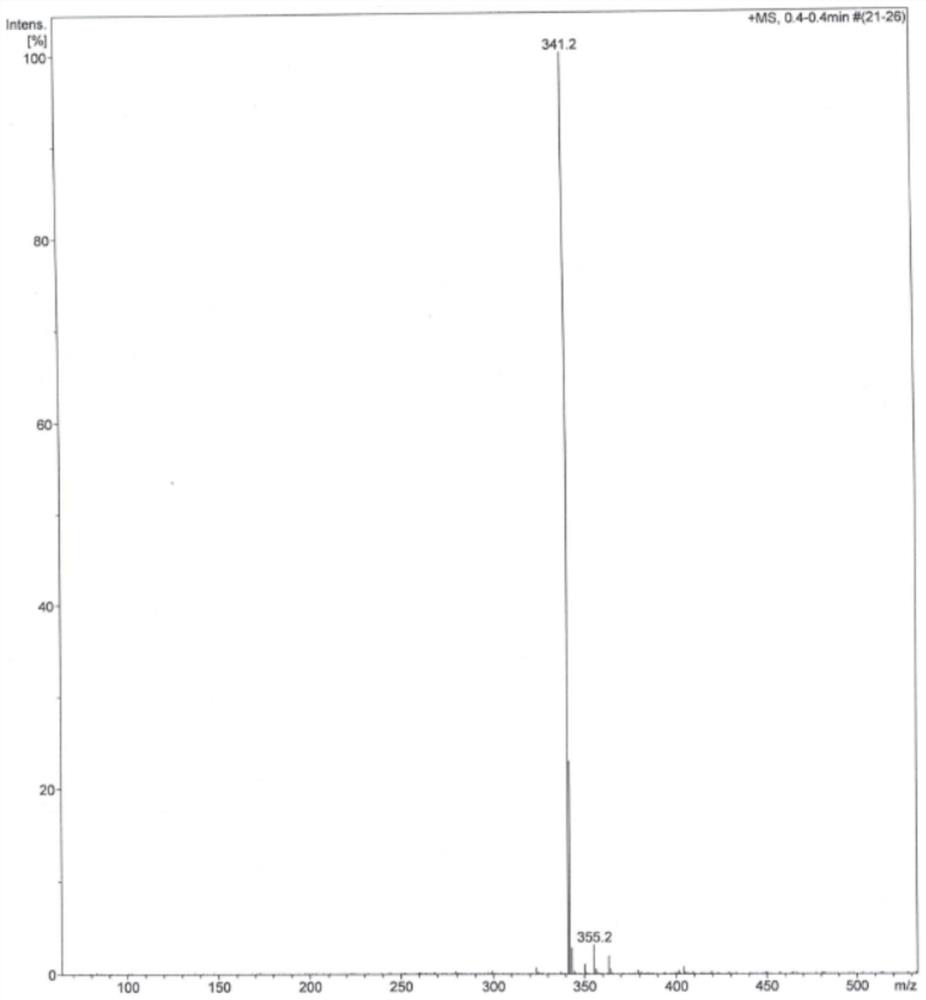
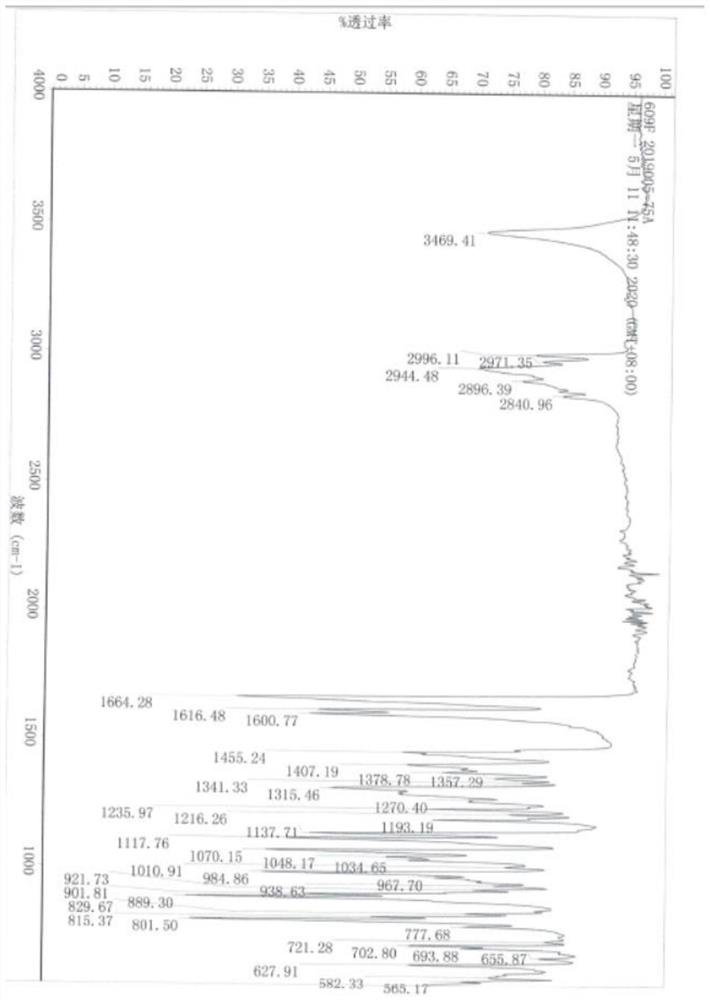
[0068] Under the protection of an inert gas, add 43.2g trimethyl phosphite to the reaction liquid, react for 8 hours at a reaction temperature of 50°C, then cool the temperature of the reaction liquid to 10°C, add 2000g of water to the reaction liquid to make the crystal Precipitated, filtered to obtain a solid product, washed the solid product with 100 mL of methanol at 0° C., and dried to obtain 79.0 g of betamethasone rearrangement (III), with a molar yield of 96.0%.
Embodiment 2
[0070] Under the protection of an inert gas, 100g of compound (I), 600mL of methanol, 100mL of DMSO, and 62.8g of sodium methoxide were mixed uniformly, and reacted for 1 hour at a reaction temperature of 40°C to obtain a reaction solution containing compound (II);
[0071] Under the protection of an inert gas, add 86.4g trimethyl phosphite to the reaction liquid, and react for 4 hours at a reaction temperature of 40°C, then cool the temperature of the reaction liquid to 20°C, add 1000g of water to the reaction liquid to make the crystal Precipitated, filtered to obtain a solid product, washed the solid product with 50 mL of methanol at 0° C., and dried to obtain 79.2 g of betamethasone rearrangement (III), with a molar yield of 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com