Method for synthesizing 5-bromo-2-methoxyphenol
A technology of o-methoxyphenol and methoxybenzene carbonate, applied in the chemical field, can solve the problems of expensive raw materials, harsh production conditions, and low selectivity in the bromination process, and achieve complete conversion of raw materials, mild reaction conditions, The effect of strong application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The substantive content of the present invention will be described in detail below in conjunction with the embodiments, but the protection scope of the present invention is not limited thereto.
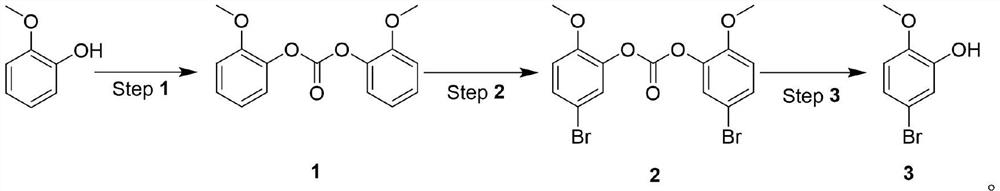
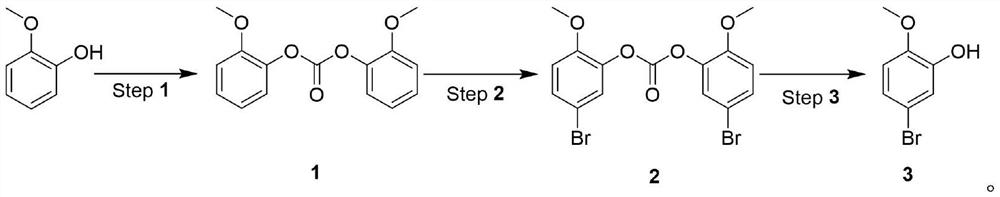
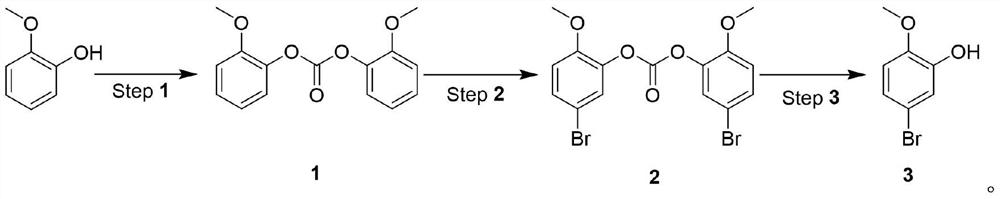
[0027] Under the catalysis of organic amine, react with carbonate and o-methoxyphenol to generate o-methoxybenzene carbonate, then react with NBS to generate 5-bromo-2-methoxybenzene carbonate, and finally obtain 5- Bromo-2-methoxyphenol.
[0028] The synthetic route is as follows:
[0029]
[0030] The preparation process comprises the following steps:
[0031] 1. Synthesis of o-methoxybenzene carbonate (1):
[0032] Add 100g (1 equivalent) of o-methoxyphenol, 120g (0.5 equivalent) of trichloromethyl carbonate, and 800mL of dichloromethane into a 2L three-necked flask, and slowly add 87g (1.05 equivalent) of triethyl amine. After the dropwise addition was completed, the mixture was stirred at room temperature for 5 hours. The reaction generates a large amount of solids...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com