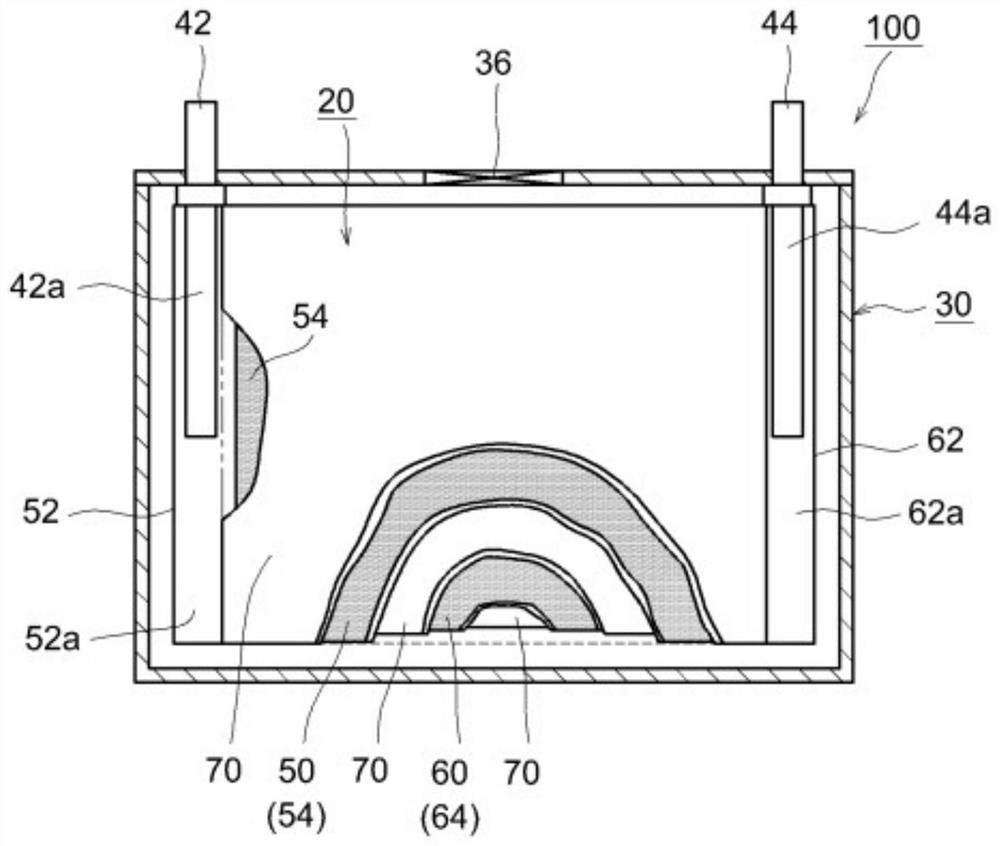
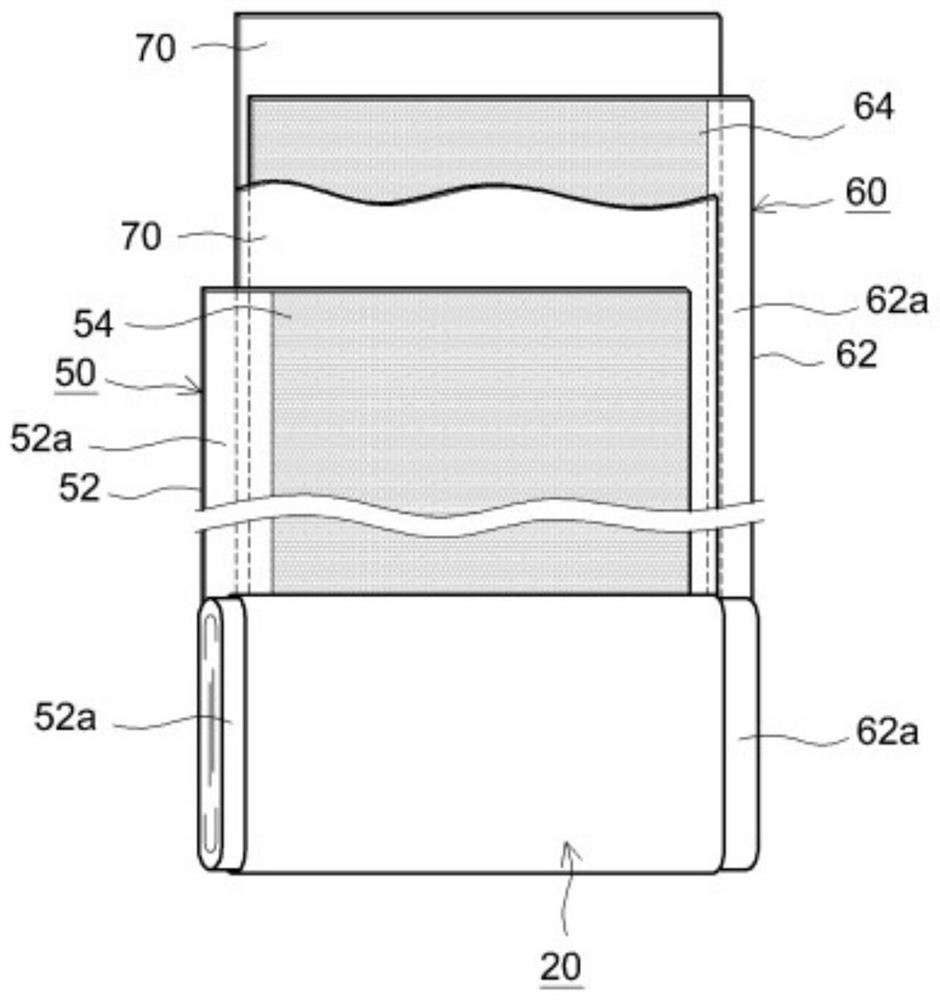
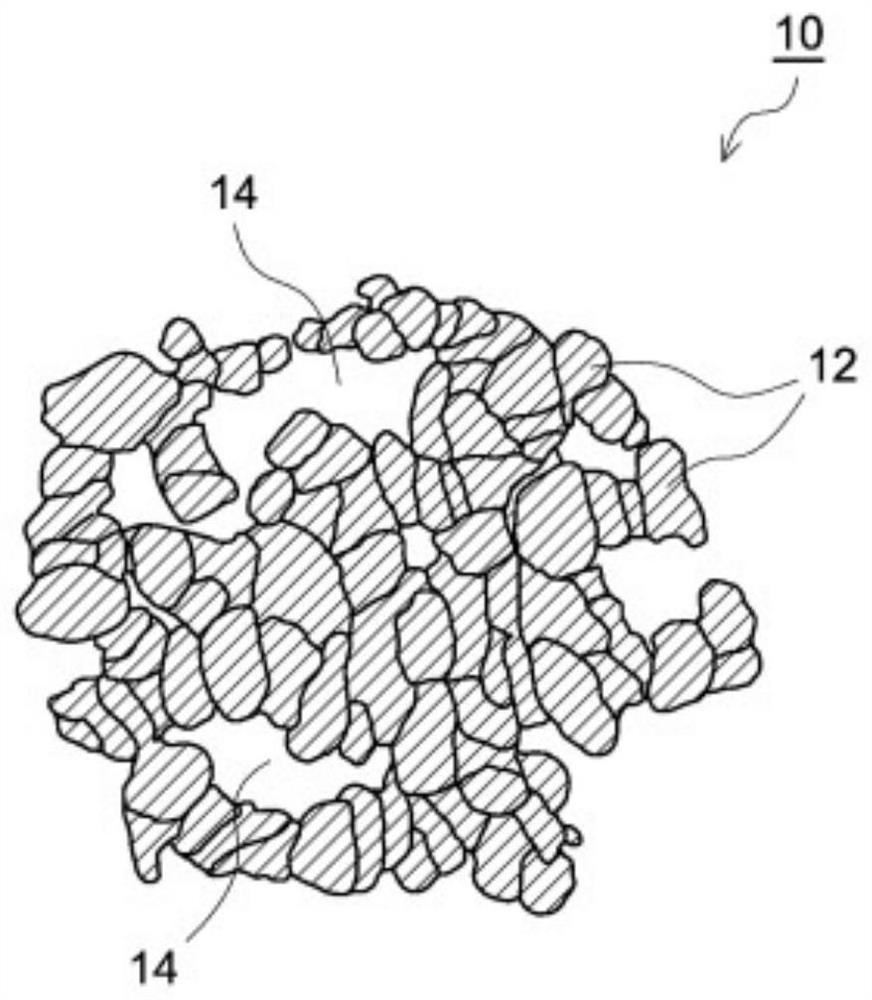
Non-aqueous electrolyte secondary battery
A non-aqueous electrolyte, secondary battery technology, applied in non-aqueous electrolyte storage batteries, secondary batteries, batteries, etc., can solve problems such as small pores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7、 comparative example 1~5
[0104]
[0105] Dissolve nickel sulfate, cobalt sulfate, and manganese sulfate in water at a molar ratio of 5:2:3 to prepare a mixed solution containing metal components. On the other hand, a reaction liquid whose pH was adjusted using sulfuric acid and ammonia water was prepared in the reaction container. In addition, a pH adjustment solution obtained by mixing an aqueous sodium carbonate solution and an aqueous ammonium carbonate solution was prepared.
[0106] While controlling the pH with the pH adjusting liquid, the mixed liquid containing the metal component was added to the reaction liquid at a predetermined speed with stirring. After a predetermined time elapses, crystallization is completed. The crystals were washed with water, and then filtered and dried to obtain hydroxide particles, ie precursor particles. At this time, the pores formed in the precursor particles are controlled by changing the addition rate, pH, stirring rate, and reaction time of the mixed sol...
Embodiment 8~10、 comparative example 6~9
[0119]Precursor particles were produced in the same manner as above except that nickel sulfate, cobalt sulfate, and manganese sulfate were used in a molar ratio of 8:1:1. At this time, the pores formed in the precursor particles are controlled in the same manner as described above.
[0120] The obtained precursor particles and lithium carbonate were mixed so that the molar ratio of lithium to the total of nickel, cobalt, and manganese became 1.05. The mixture was calcined at 800° C. for 10 hours to obtain a lithium composite oxide (Li 1.05 Ni 0.8 co 0.1 mn 0.1 o 2 ).
[0121] The obtained lithium composite oxide was added to a mixed solution containing a metal component obtained by dissolving nickel sulfate, cobalt sulfate, and manganese sulfate in water at a molar ratio of 8:1:1, and obtained in the same manner as above except that A lithium composite oxide having a rock-salt structure layer on the surface. At this time, the thickness of the rock-salt structure layer i...
Embodiment 11~13、 comparative example 10~13
[0126] Precursor particles were produced in the same manner as above except that nickel sulfate, cobalt sulfate, and manganese sulfate were used in a molar ratio of 4:3:3. At this time, the pores formed in the precursor particles are controlled in the same manner as described above.
[0127] The obtained precursor particles and lithium carbonate were mixed so that the molar ratio of lithium to the total of nickel, cobalt, and manganese became 1.05. The mixture was fired at 950° C. for 10 hours to obtain a lithium composite oxide (Li 1.05 Ni 0.4 co 0.3 mn 0.3 o 2 ).
[0128] The obtained lithium composite oxide was added to a mixed solution containing a metal component obtained by dissolving nickel sulfate, cobalt sulfate, and manganese sulfate in water at a molar ratio of 4:3:3, and obtained in the same manner as above except that A lithium composite oxide having a rock-salt structure layer on the surface. At this time, the thickness of the rock-salt structure layer is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com