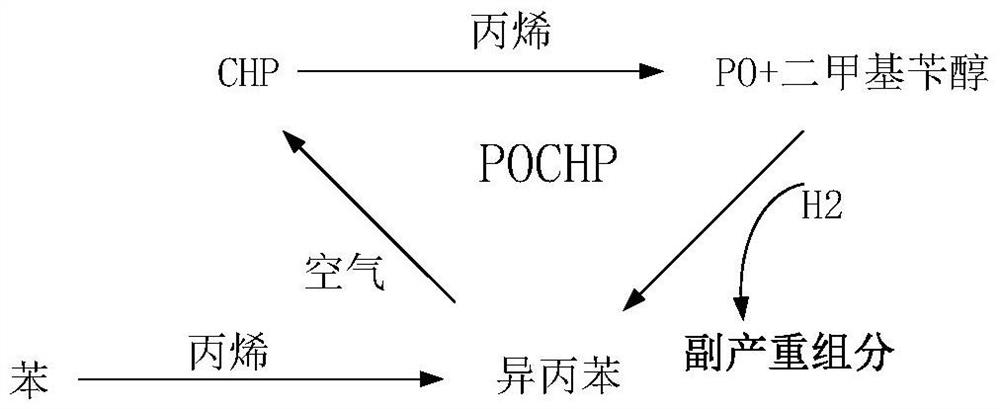
Method for reducing unit consumption of cumene in epoxypropane production process using cumene co-oxidation method
A technology of propylene oxide and co-oxidation method, which is applied in the production of bulk chemicals, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of inability to expand large-scale, increase of unit consumption of cumene, and increase of cumene loss and other issues, to achieve the effect of improving technical economy, reducing unit consumption, and reducing unit consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation of palladium / foam carbon catalyst in the embodiment:
[0045] Foamed carbon prepared from coal and coal series as the carrier: take 99.5 g of the prepared foamed carbon and put it into a three-necked bottle, add a small amount of distilled water to disperse. The chloropalladium acid solution containing 0.5gPd was added to a three-necked flask under the condition of stirring at 40°C in a water bath for adsorption. After the adsorption was completed, excess formaldehyde was added to a water bath at 60°C for reduction for 6 hours. A foamed carbon-supported Pd catalyst A is obtained, and the loading amount of Pd is 0.5%.
[0046] Catalyst B with Pd loading of 0.1% was prepared by changing the loading of support and chloropalladium acid.
Embodiment 1
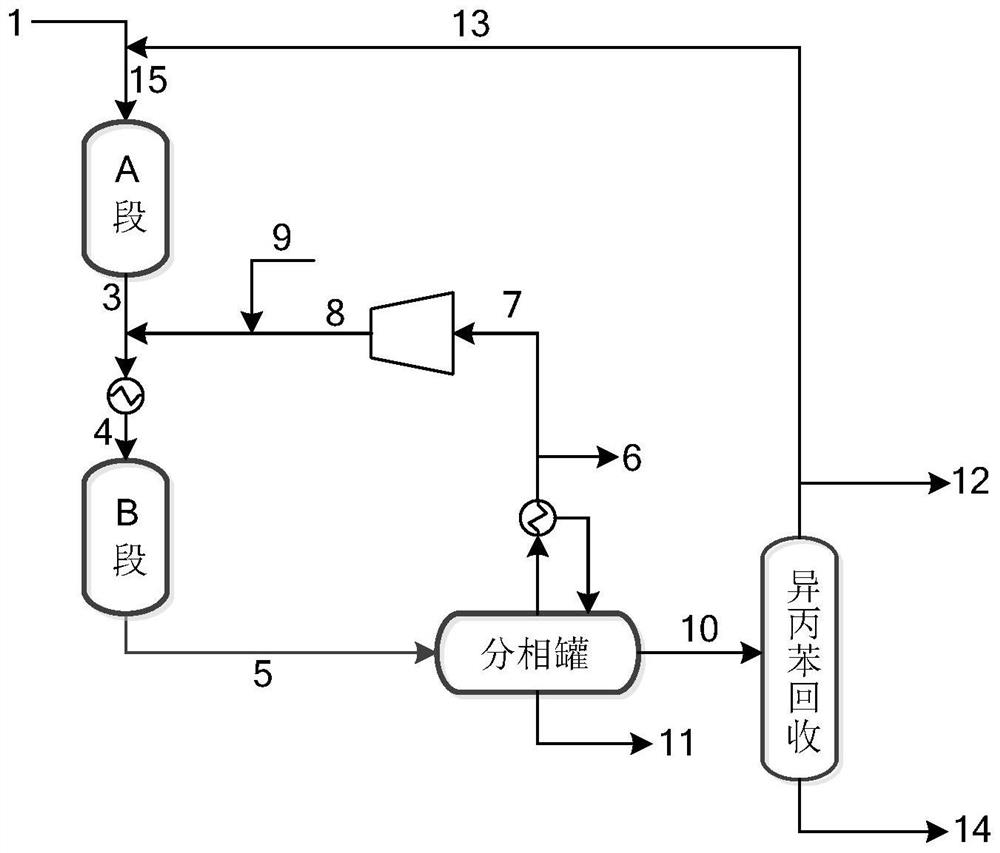
[0048] In the cumene heavy component stream 1 obtained after rectification recovery of cumene in the dimethyl benzyl alcohol hydrogenolysis reaction liquid, containing 9.0% cumene, 21.0% dimethyl benzyl alcohol, 24.0% 2 - Phenyl-n-propanol, 45% cumene.
[0049] The hydrogenation reactor was filled with catalyst A with 0.5% Pd supported on foamed carbon.
[0050] (a) Cumene heavy component stream 1 enters from the upper end of the cracking reactor, the temperature of the cracking reactor is 300°C, the reaction pressure is 1.0MPaG, and the volume space velocity is 2.0h -1 , wherein heavy components such as cumene undergo rapid cracking reactions to generate stream 3 containing cumene and α-methylstyrene (AMS);
[0051] (b) stream 3 obtains stream 4 after being cooled to 50 DEG C, and stream 4 enters the hydrogenation reactor of packing palladium / foam carbon catalyst A together with circulating hydrogen stream 8 and fresh hydrogen, and α- Methyl styrene and dimethyl benzyl alco...
Embodiment 2~9
[0055] The difference with embodiment 1 is:
[0056] Change the pressure, temperature and space velocity of the reactor. The hydrogenation catalyst used was Catalyst B. The specific reaction conditions and results are shown in Table 2.
[0057] Table 2 Embodiment 1~9 result
[0058]
[0059] The conversion rates of dimethyl benzyl alcohol and cumene in the above table represent the total reaction effect after passing through the cracking reactor and the hydrogenation reactor. Wherein the dimethyl benzyl alcohol conversion test method: measure the mass concentration of the dimethyl benzyl alcohol of stream 1, stream 10 respectively, calculate according to the following formula,
[0060]
[0061] Where n is the mass flow ratio of stream 10 and stream 1, which can be simply calculated according to the following formula, and n≈3 in Examples 1-9. Since a small amount of water is generated during the cracking reaction, the flow ratio calculated by the following formula wil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com