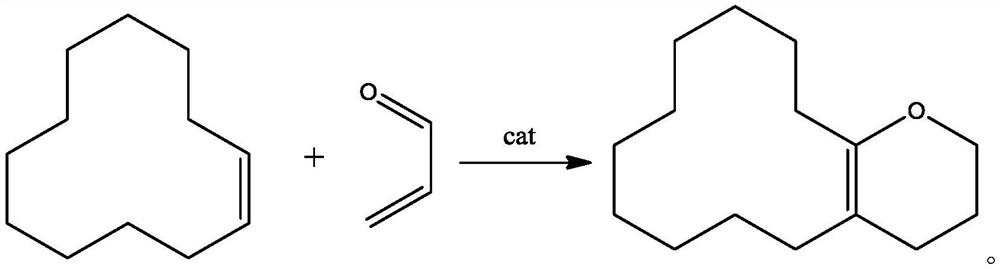
Method for preparing oxabicyclohexadecene
An oxabicyclo and hexadecene technology is applied in the field of preparation of oxabicyclohexadecene, and can solve the problems of large amount of waste water, low nucleophilic substitution reaction yield, difficulty in purification and post-treatment, and the like, and achieves low cost of raw materials , the process route is simple, the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of supported organometallic catalysts:
[0036] Mix 200.75g of rhodium acetate and 204.01g of 2,6-bisdiphenylphosphinopyridine in 800g of distilled water, raise the temperature to 60°C and stir for 4 hours; add 178.59g of 4A molecular sieve under stirring to obtain a slurry;
[0037] Use 25wt% sodium bicarbonate solution as the alkaline precipitant, heat the slurry and the alkaline precipitant to 50°C respectively, and slowly add the alkaline precipitant to the slurry until the pH of the system is 10.0, and control the reaction temperature of the precipitation process 50°C; After the precipitation reaction is completed, the slurry is obtained after aging at 70°C for 6 hours;
[0038] Then the slurry was fully filtered, the surface of the filter cake was washed with deionized water, and the filter cake was dried at 90°C for 9 hours to obtain the dried filter cake, which was then calcined at 190°C for 6 hours, crushed and pressed into tablets to obtain the cat...
Embodiment 2
[0042] Preparation of supported organometallic catalysts:
[0043] Mix 311.27g of rhodium chloride and 255.13g of 1-(2-diphenylphosphine-1-naphthalene)isoquinoline in 800g of distilled water, raise the temperature to 60°C and stir for 4 hours; add 102.05g of 4A molecular sieve under stirring to obtain a slurry ;
[0044] Use 25wt% sodium bicarbonate solution as the alkaline precipitant, heat the slurry and the alkaline precipitant to 50°C respectively, and slowly add the alkaline precipitant to the slurry until the pH of the system is 10.0, and control the reaction temperature of the precipitation process 50°C; After the precipitation reaction is completed, the slurry is obtained after aging at 70°C for 6 hours;
[0045] Then the slurry was fully filtered, the surface of the filter cake was washed with deionized water, and the filter cake was dried at 90°C for 9 hours to obtain the dried filter cake, which was then calcined at 190°C for 6 hours, crushed and pressed into table...
Embodiment 3
[0050] Preparation of supported organometallic catalysts:
[0051] Mix 300.9g of rhodium chloride and 209.2g of 2-(diphenylphosphine)methylpyrrolidine in 800g of distilled water, raise the temperature to 60°C and stir for 4 hours; add 153.07g of ordered mesoporous carbon under stirring to obtain a slurry;
[0052] Use 25wt% ammonium carbonate solution as an alkaline precipitant, heat the slurry and the alkaline precipitant to 50°C, and slowly add the alkaline precipitant to the slurry until the pH of the system is 11.0, and control the reaction temperature of the precipitation process to 50°C °C; After the precipitation reaction is completed, the slurry is obtained after aging at 70 °C for 6 hours;
[0053] Then the slurry was fully filtered, the surface of the filter cake was washed with deionized water, and the filter cake was dried at 100°C for 10 hours to obtain the dried filter cake, which was then calcined at 180°C for 8 hours, crushed and pressed into tablets to obtain th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com