Stainless foil current collector for secondary battery positive electrodes
A secondary battery, stainless steel foil technology, applied in the field of current collectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Ferritic stainless steel having the composition shown in Table 2 was smelted, subjected to normal hot rolling, pickling, cold rolling, and annealing, and then normal foil rolling (rolled material (hard material)) to produce a plate with a thickness of 10 μm stainless steel foil, prepare the specimen.
[0114] The surface hardness of the obtained stainless steel foil was calculated|required by Vickers hardness based on JIS Z 2244:2009. At this time, the test force (indentation load of the indenter) was set to 5 gf.
[0115] The corrosion resistance was evaluated by immersing a stainless steel foil test piece in an electrolyte close to the positive electrode environment and measuring the anode current. The electrolyte uses 1 mol / L (L refers to liter.) LiPF dissolved in a non-aqueous solvent (mixing ethylene carbonate and ethyl methyl carbonate at a volume ratio of 1:3) as an electrolyte 6 the resulting solution. Make a sample of stainless steel foil (thickness 10 μ m ×...
Embodiment 2
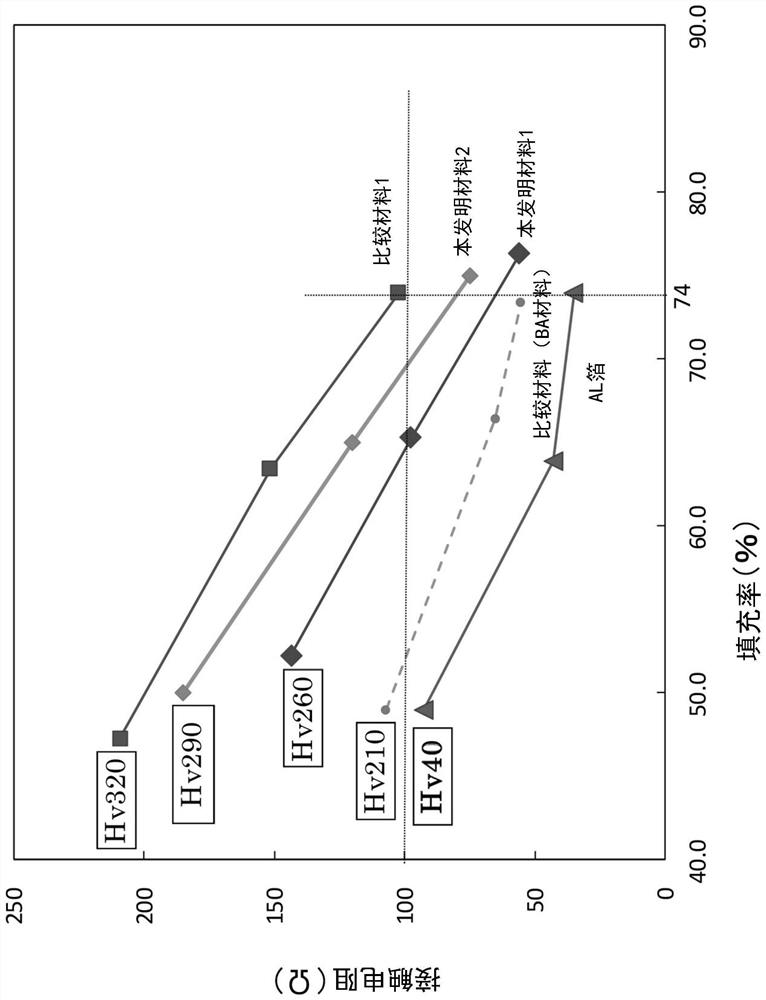
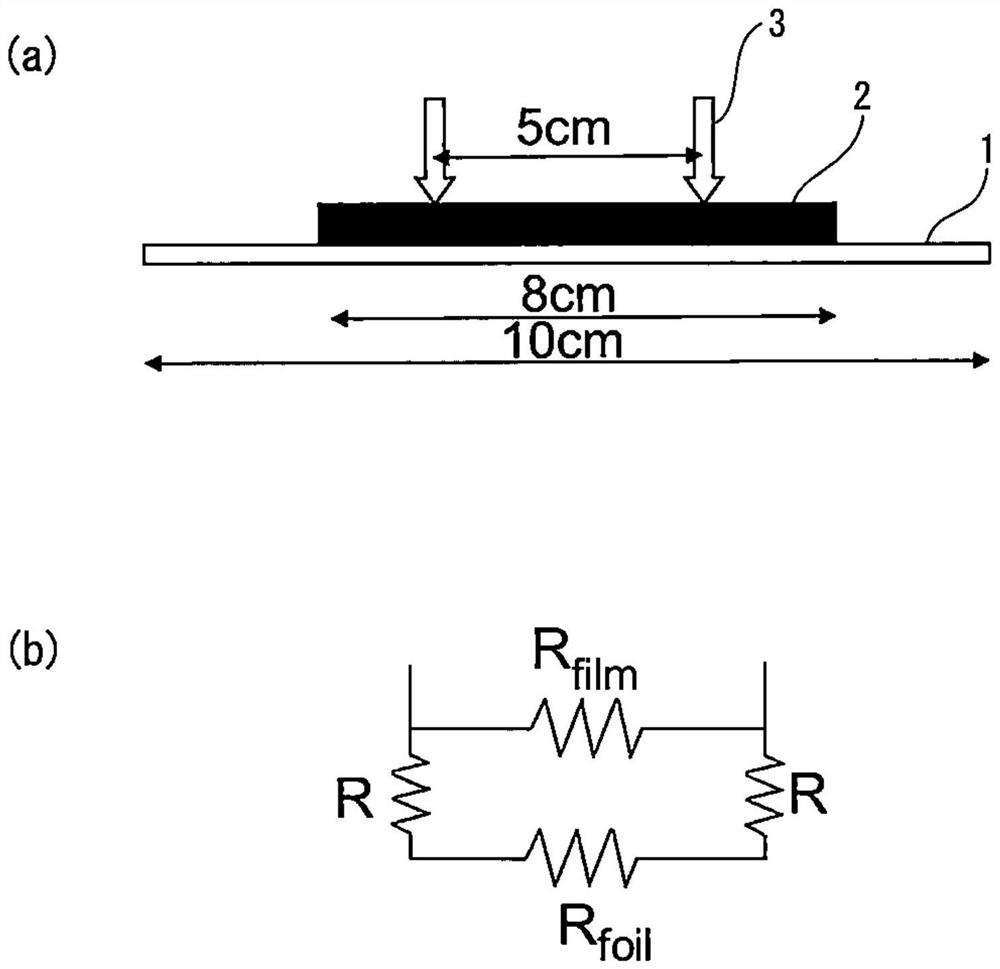
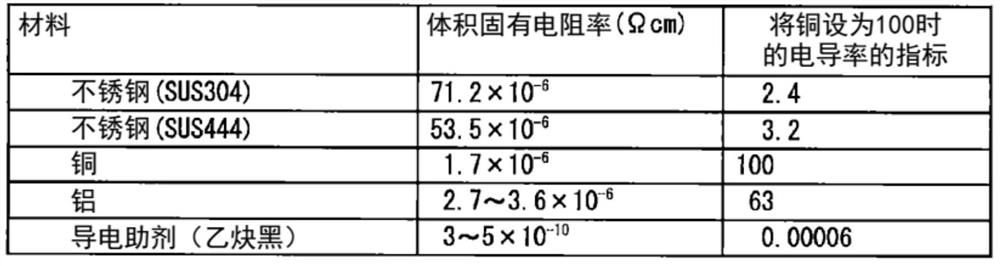
[0123] The filling rate of the positive electrode mixture and the electrical contact resistance with the stainless steel foil were measured in the positive electrode current collector obtained by coating the positive electrode mixture on the surface of the stainless steel foil and pressing it.
[0124] Stainless steel foil (rolled state material (hard material)) uses 1 steel in Table 2 ( figure 1 The inventive material 1) and 2 steel ( figure 1 The present invention material 2) and 7 steel ( figure 1 Comparative material 1). Stainless steel foils each having a plate thickness of 10 μm were used. For steel 7, steel subjected to BA treatment (annealing temperature 500°C) was used in order to change the surface hardness ( figure 1 The comparative material (BA material)) and steel without BA treatment (rolled state material (hard material)) ( figure 1 Comparative material 1). For comparison, Al foil (thickness: 20 μm) ( figure 1 Al foil). The surface hardness of each materi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com