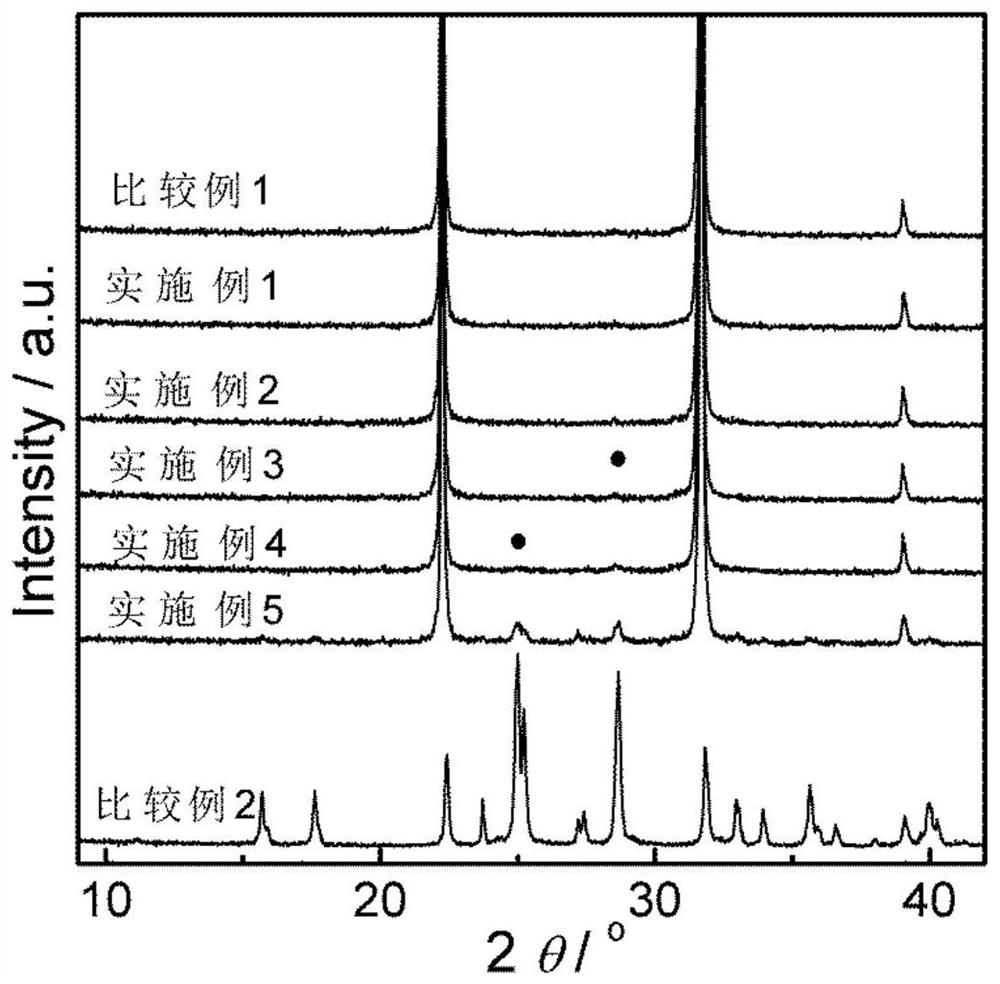
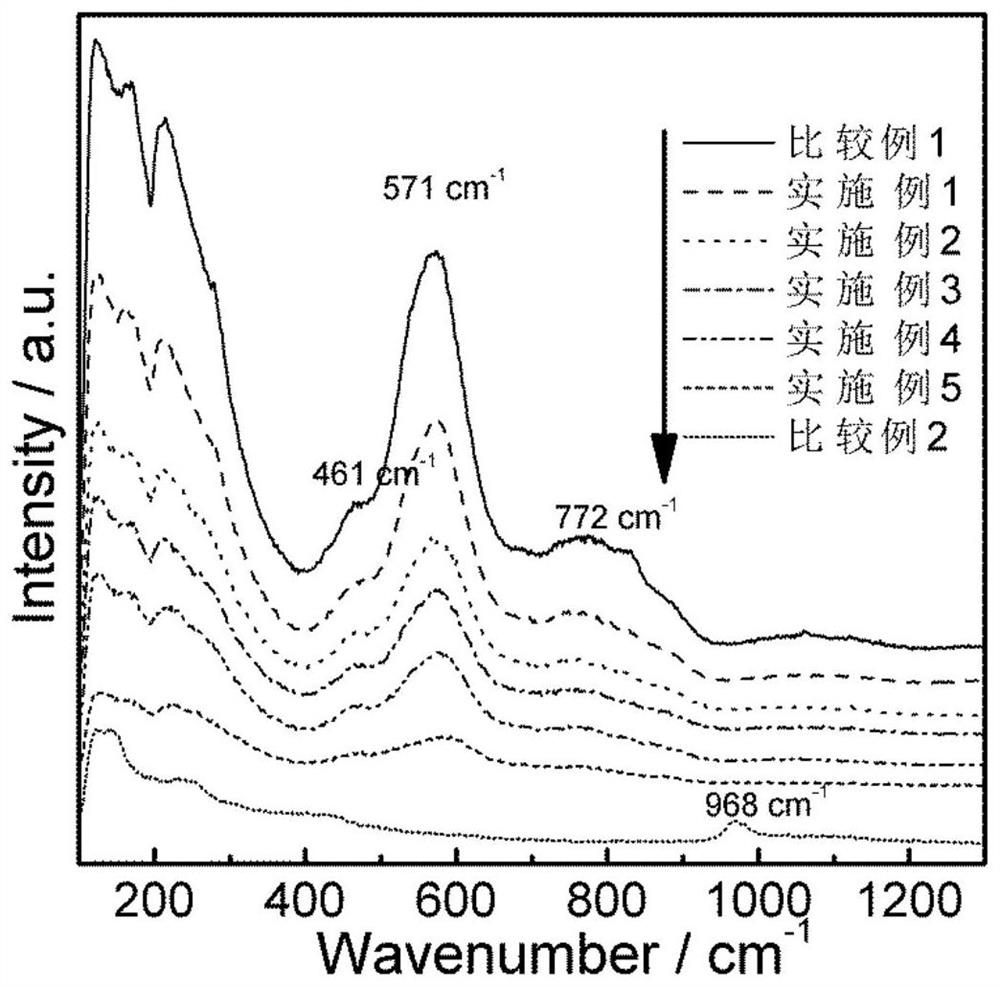
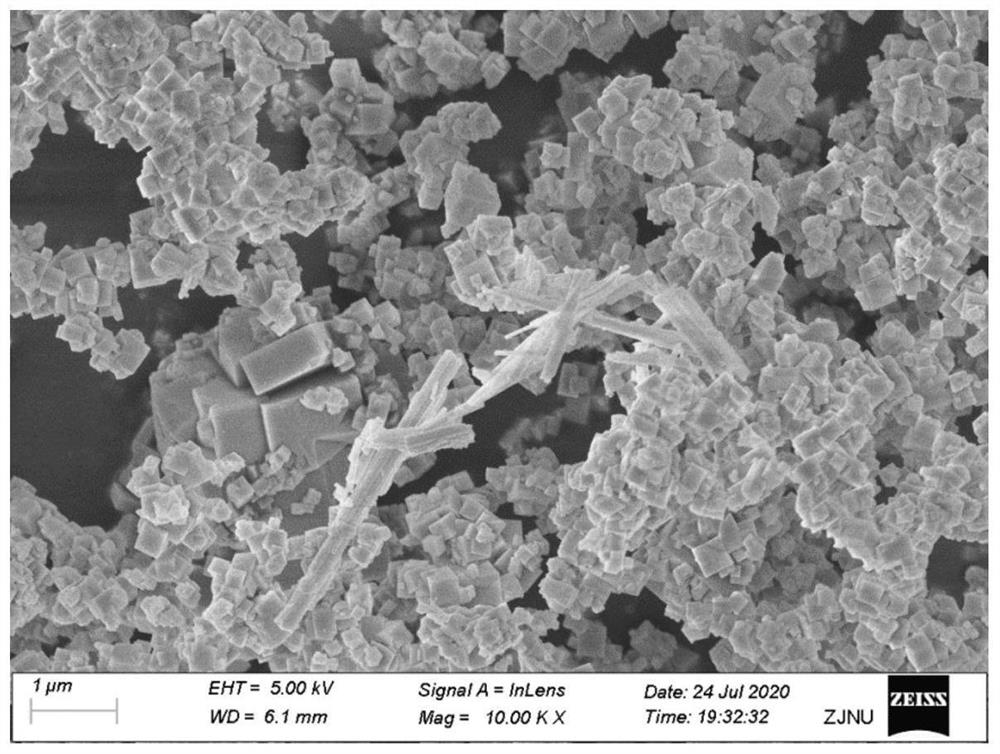
Bismuth sulfide composite potassium tantalate niobate catalyst as well as preparation method and application thereof
A technology of potassium tantalum niobate and catalyst is applied in the field of bismuth sulfide composite potassium tantalum niobate catalyst and the preparation field of the catalyst, and achieves the effects of excellent photocatalytic and piezoelectric catalytic ammonia production performance and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a preparation method of bismuth sulfide composite potassium tantalum niobate, comprising:
[0040] (1) Weigh 44.80g KOH solid with an analytical balance and dissolve it in 40mL of ultrapure water, then weigh 3.315g (0.0075mol) Ta 2 o 5 and 0.665g (0.0025mol) Nb 2 o 5 Slowly put it into the above KOH solution, stir it magnetically for 1 hour, transfer it to the inner liner made of 100mL PPL, add ultrapure water to make the total volume of the suspension 80mL, stir it evenly with a glass rod, and put it into a hydrothermal reaction kettle. Hydrothermal reaction at 200°C for 24h. After the reaction is completed and cooled to room temperature, pour off the supernatant, wash with a mixed solution of ultrapure water and alcohol for 3 times; put it in an oven, and dry it at 60°C for 12 hours to obtain white KTa 0.75 Nb 0.25 o 3 solid solution.
[0041] (2) Weigh 9.6027gNa 2 S·9H 2 O, respectively configured as 100mL of Na 2 S solution (0.40mo...
Embodiment 2
[0043] (1) With the step of (1) in embodiment 1.
[0044] (2) Weigh 1.23g (0.005mol) KTa 0.75 Nb 0.25 o 3 Put the powder into a 100mL beaker filled with 55mL of ultrapure water, ultrasonicate at 120W for 30min to form a uniform dispersion, and pipette 0.25mL of the aforementioned Na 2 S solution was added dropwise to the suspension, magnetically stirred evenly, and then 0.0121gBi(NO 3 ) 3 ·5H 2 O crystals were magnetically stirred for 40 min, transferred to a 100 mL polytetrafluoroethylene-lined hydrothermal reactor, and hydrothermally heated at 120 °C for 12 h. After the reaction was completed and cooled to room temperature, the supernatant was discarded, washed with a mixed solution of ultrapure water and alcohol for 4 times, placed in a vacuum oven, and dried at 60°C for 16 hours in a vacuum environment to obtain the target product 0.25% Bi 2 S 3 / KTN.
Embodiment 3
[0046] (1) With the step of (1) in embodiment 1.
[0047] (2) Weigh 1.23g (0.005mol) KTa 0.75 Nb 0.25 o 3 Put the powder into a 100mL beaker filled with 55mL of ultrapure water, ultrasonicate at 120W for 30min to form a uniform dispersion, and pipette 0.5mL of the aforementioned Na 2 S solution was added dropwise to the suspension, magnetically stirred evenly, and then 0.0243gBi(NO 3 ) 3 ·5H 2 O crystals were magnetically stirred for 40 min, transferred to a 100 mL polytetrafluoroethylene-lined hydrothermal reactor, and hydrothermally heated at 120 °C for 12 h. After the reaction was completed and cooled to room temperature, the supernatant was discarded, washed with a mixed solution of ultrapure water and alcohol for 4 times, placed in a vacuum oven, and dried at 60°C for 16 hours in a vacuum environment to obtain the target product 0.5% Bi 2 S 3 / KTN.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com