Pharmaceutical composition suitable for primary treatment of extra-short-range treatment of smear-positive pulmonary tuberculosis
A composition, tuberculosis technology, applied in the field of biomedicine, can solve the problem of not finding a drug composition, etc., and achieve the effect of shortening the treatment time, low recurrence rate, and low adverse reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, be applicable to the composition of the pharmaceutical composition that treats newly diagnosed smear-positive pulmonary tuberculosis by 4.5 months chemotherapy regimen
[0031] The pharmaceutical composition provided by the present invention that is applicable to the treatment of newly diagnosed smear-positive pulmonary tuberculosis by 4.5 months chemotherapy regimen consists of levofloxacin, isoniazid, rifampicin, pyrazinamide and ethambutol, according to the unit dose (single day) Dosage) for packaging, specifically as follows (1) or (2):
[0032] (1) The pharmaceutical composition of the unit dose (single daily dose) contains 1 part of levofloxacin with a specification of 600 mg, 1 part of isoniazid with a specification of 300 mg, 1 part of rifampicin with a specification of 450 mg, and 3 parts of pyridoxine with a specification of 500 mg. Azinamide and 1 serving of ethambutol in a strength of 750 mg. It is suitable for newly diagnosed smear-positive p...
Embodiment 2
[0034] The specific application of pharmaceutical composition in embodiment 2, embodiment 1
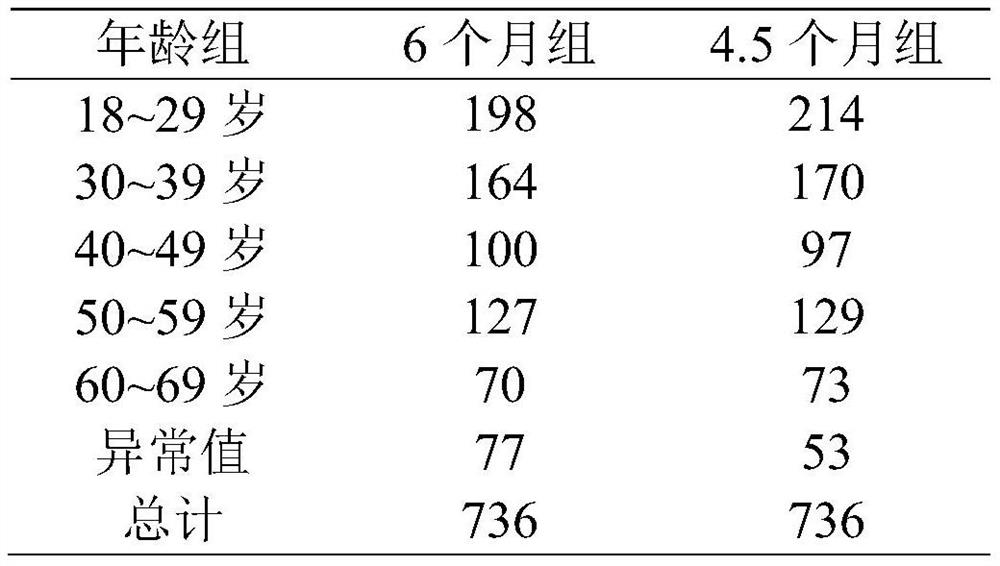
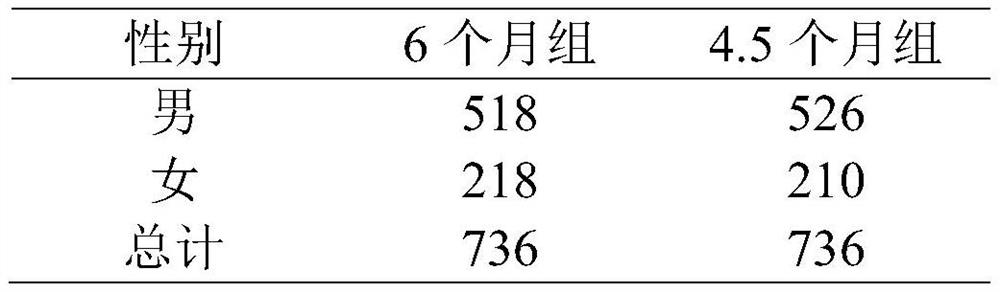
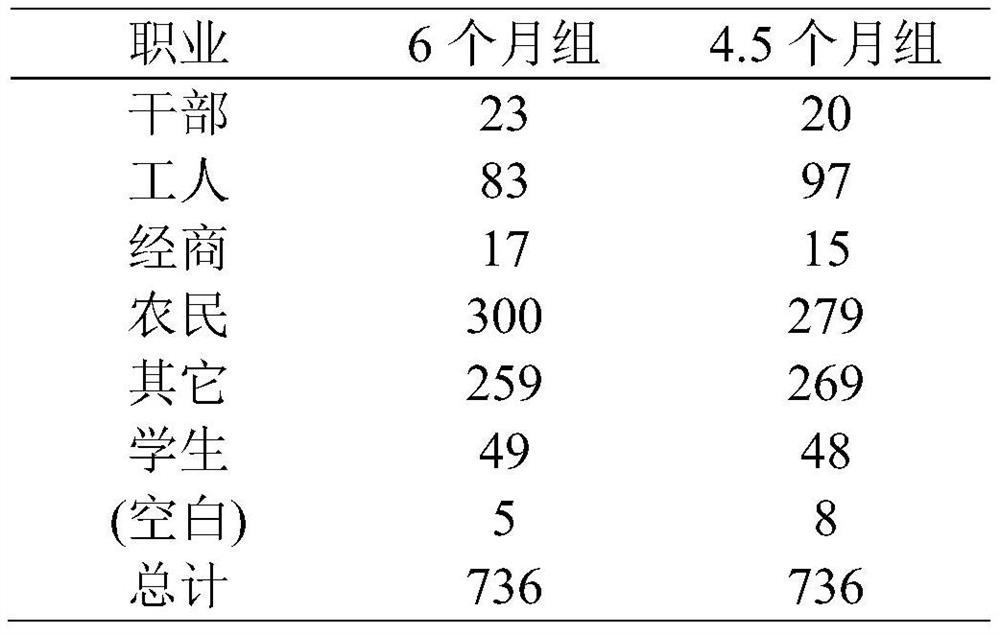
[0035] 1. Subject population
[0036] 1. Conditions for using this program
[0037] (1) Sign the informed consent;
[0038] (2) Age 18-65;
[0039] (3) The sputum smear is positive twice or the culture is positive, and the chest X-ray shows that there are active tuberculosis lesions in the lungs;
[0040] (4) Never received anti-tuberculosis treatment or anti-tuberculosis treatment for less than 1 month;
[0041] (5) Relevant laboratory examinations shall be carried out before enrollment. Among them, ALT and total bilirubin were less than 2 times the upper limit of normal value. Creatinine clearance greater than 30ml / s / min. HB is greater than 7.0 g / dL. Platelets greater than 50×10 9 / L;
[0042] (6) Women of childbearing age have a negative urine pregnancy test and promise to take contraceptive measures during the course.
[0043] 2. Exclusions
[0044] (1) Combined with se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com