Salt of gabapentin and 2, 6-pyridine dicarboxylic acid as well as preparation method and application thereof
A technology of dipicolinic acid and gabapentin is applied in the fields of medical application and crystallization technology, which can solve problems such as chemical instability, and achieve the effects of high purity, simple and feasible preparation method and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Under room temperature conditions, gabapentin (34.2g) and 2,6-pyridinedicarboxylic acid (33.4g) bulk drug are completely dissolved in 500ml ethanol solution, the solution is filtered and slowly volatilized to remove the solvent at room temperature to obtain gabapentin and 2 , the salt of 6-pyridinedicarboxylic acid (65.8 g).
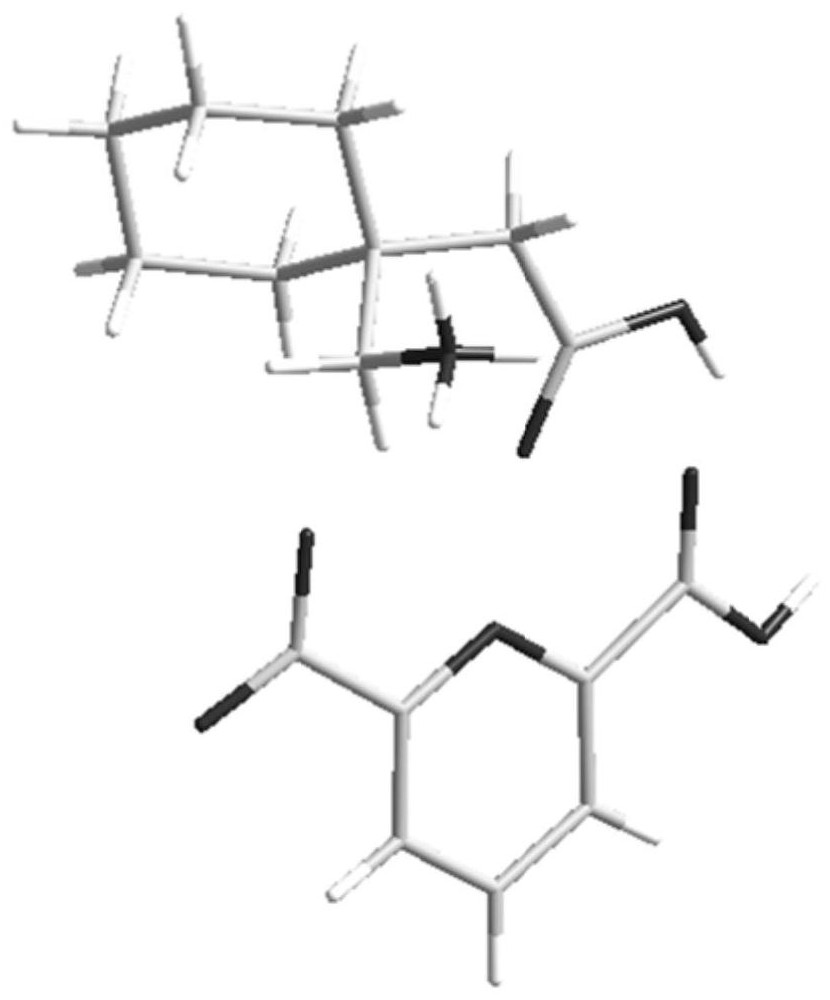
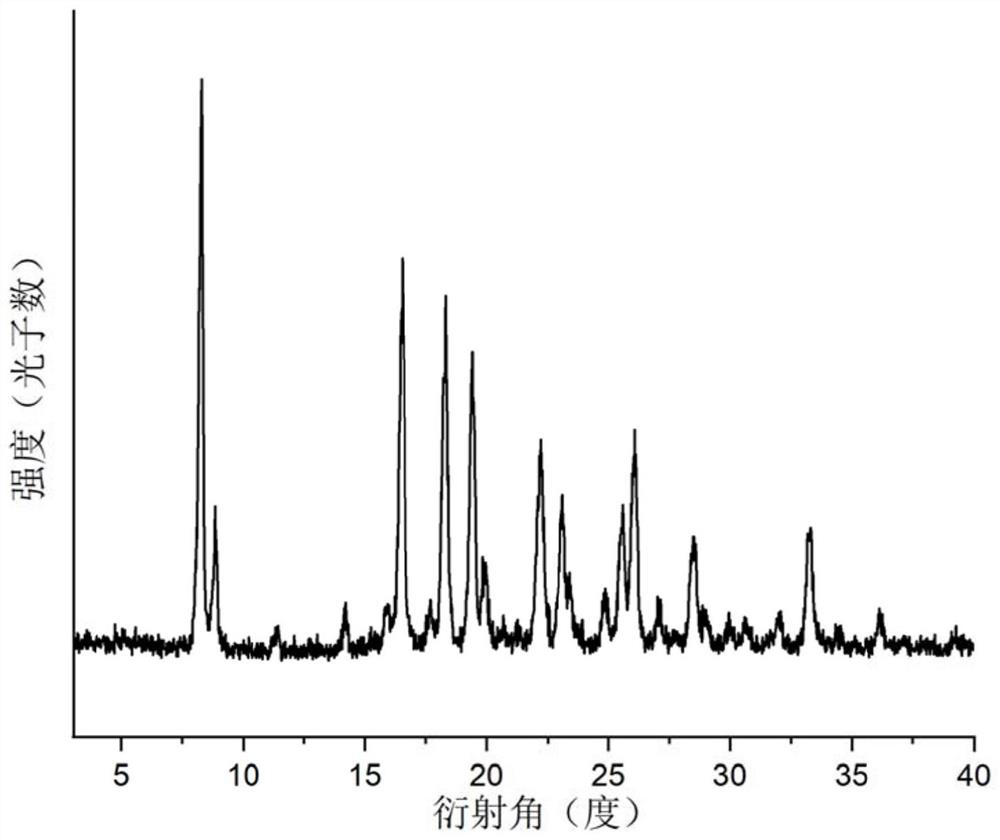
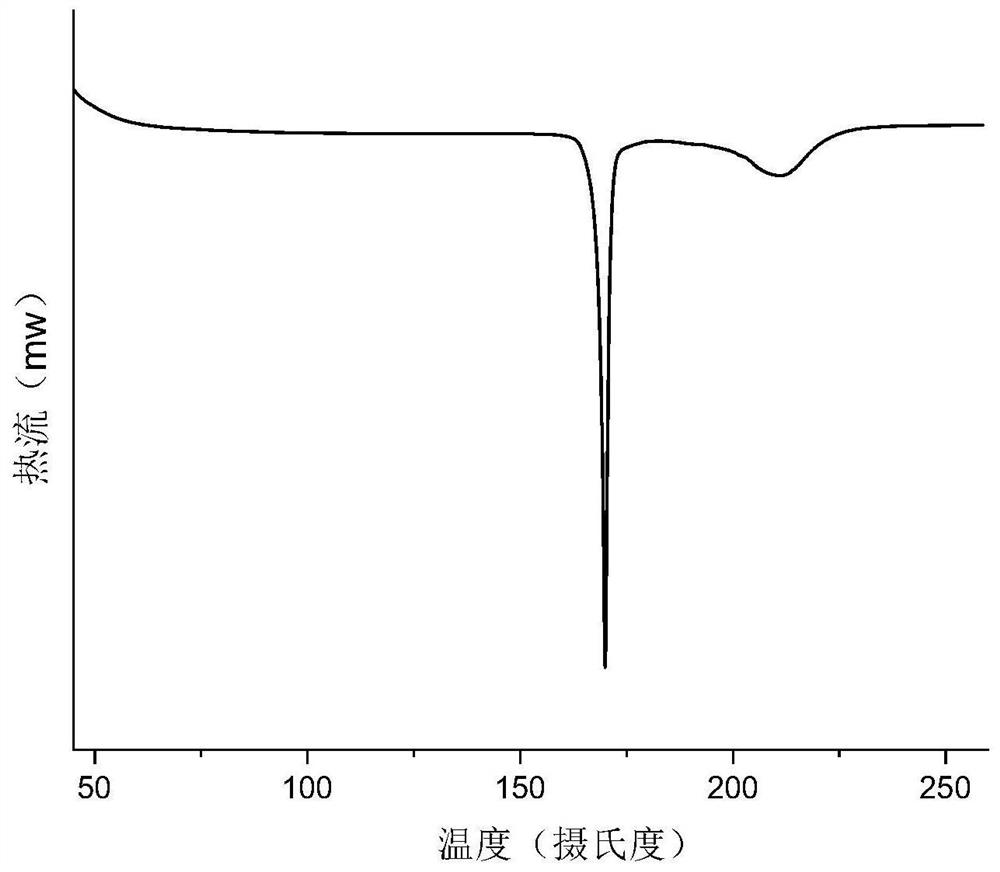
[0060] The prepared salt was subjected to solid-state characterization, Figure 1-Figure 4 Respectively show embodiment 1 gabapentin and 2, the X-ray single crystal diffraction pattern (SCXRD), X-ray powder diffraction pattern (XRPD), differential scanning calorimetry (DSC), thermal TGA.
Embodiment 2
[0062] Under room temperature conditions, gabapentin (34.2g) and 2,6-pyridinedicarboxylic acid (33.4g) were added into ethanol solvent according to the molar ratio of 1:1, until it was stirred into a suspended state at room temperature, reaction 3 day, filtered, and dried at room temperature to obtain a salt of gabapentin and 2,6-pyridinedicarboxylic acid (68.0 g).
Embodiment 3
[0064] At room temperature, gabapentin (34.2g) and 2,6-pyridinedicarboxylic acid (33.4g) were added into methanol solvent at a molar ratio of 1:1 until they were stirred into a suspension at room temperature. Reaction 3 day, filtered, and dried at room temperature to obtain a salt of gabapentin and 2,6-pyridinedicarboxylic acid (65.8 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com