Preparation method of high-purity medicinal roxatidine acetate hydrochloride
A high-purity technology for roxatidine hydrochloride acetate, applied in the field of synthesis and production of roxatidine acetate hydrochloride, can solve the problems of no impurity content, affecting the quality and yield of intermediates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of preparation method of high-purity medicinal roxatidine hydrochloride acetate of the present embodiment is carried out according to the following steps:
[0040] Step 1: Preparation of 3-(1-piperidinylmethyl)phenol
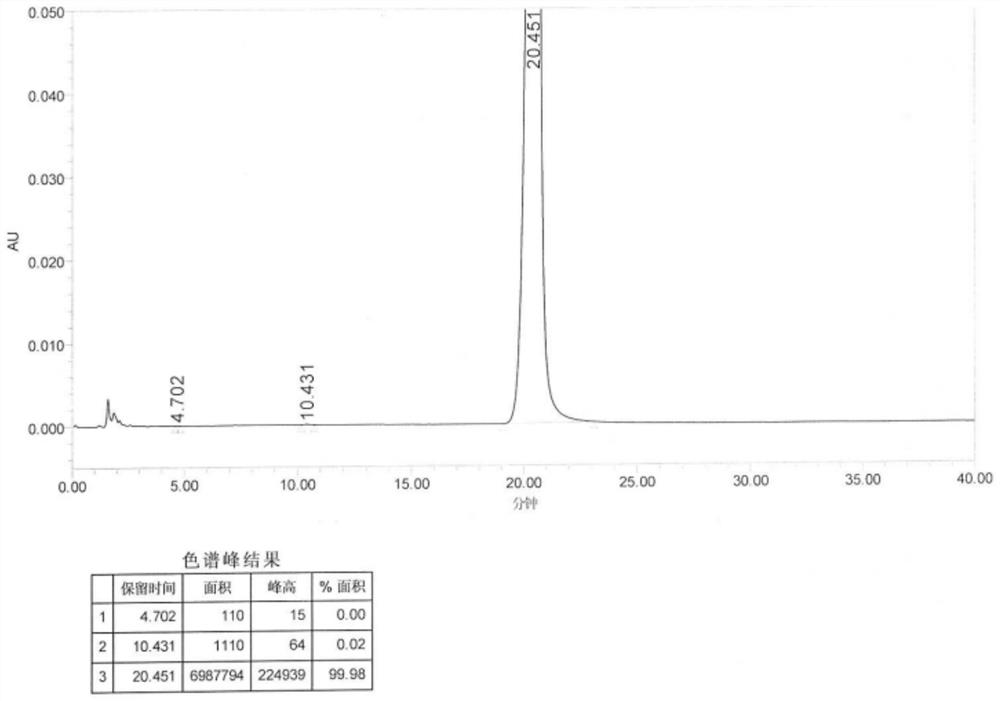
[0041]Add m-hydroxybenzaldehyde (60 g) and DMF (80 mL) into the reaction kettle, and stir. Control the temperature below 30°C, add piperidine (100mL) dropwise to the high level tank, after dropping, raise the temperature to 50°C, stir for 30min, cool down the system below 40°C, add formic acid (50mL) dropwise, add water (4mL) after dropping, and heat up to 110°C , Monitor the reaction until it stops, about 2h. Cool down to 40°C, add water and stir, add 0.25N sodium hydroxide to adjust the pH to 9.4, naturally crystallize, filter, wash with water, and dry in a vacuum oven to obtain 86.4 g of 3-(1-piperidylmethyl)phenol with a yield of 92 %, purity 99.98% (see figure 1 ).
[0042] Step 2: Preparation of 3-[3-(1-piperidinylmethyl)phenoxy]alanine ...
Embodiment 2
[0047] Step 2: 3-[3-(1-piperidinylmethyl)phenoxy]alanine
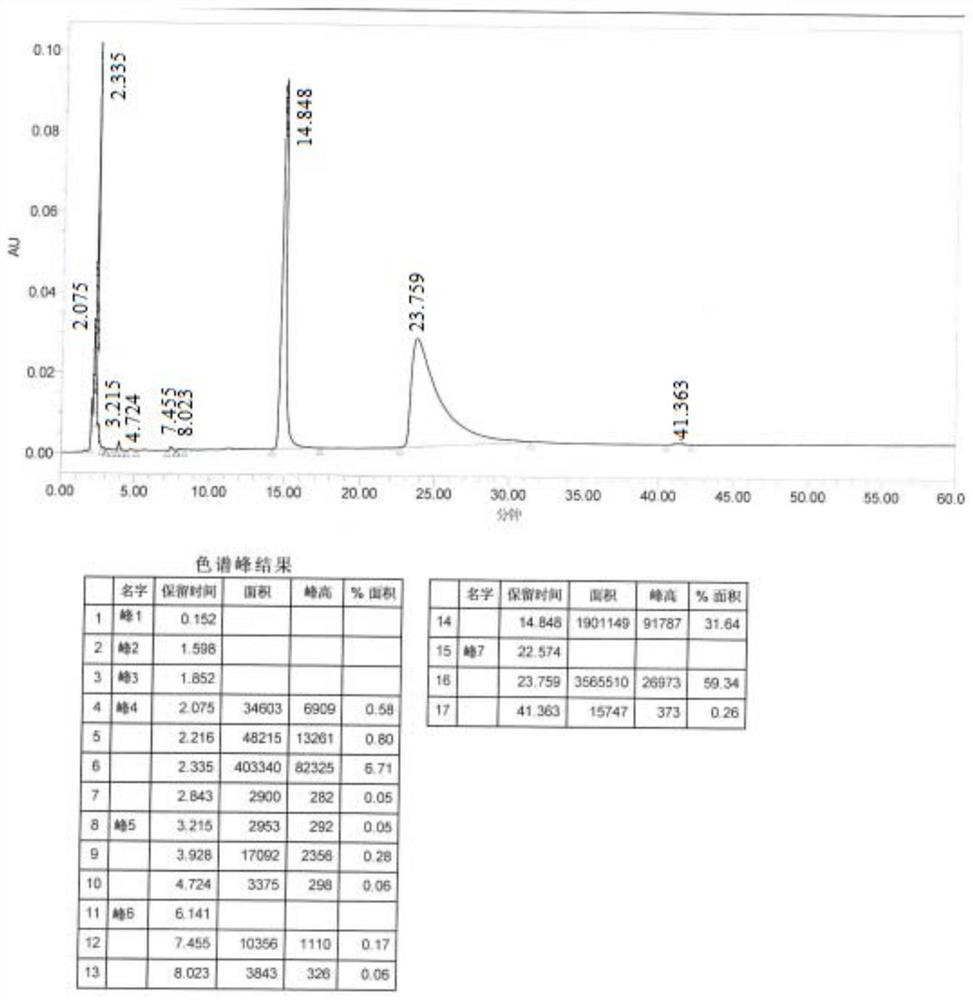
[0048] Add DMF (100mL), 3-(1-piperidinylmethyl)phenol, (48g), 3-chloropropylamine hydrochloride (40g) in the reaction kettle, stir, add sodium hydroxide (140g), be warming up to 90 °C, monitor the reaction until it stops. Filtrate, add water to the filtrate, stir for 15 min, extract twice with dichloromethane, wash the organic layer with saturated brine, add anhydrous sodium sulfate to dry, filter and evaporate the filtrate to give a light yellow oil 3-[3-(1-piperone Pyridylmethyl)phenoxy]alanamine (48.6g), about 78% yield, 97.89% purity. (See Figure 5 )
Embodiment 3
[0050] Step 2: 3-[3-(1-piperidinylmethyl)phenoxy]alanine
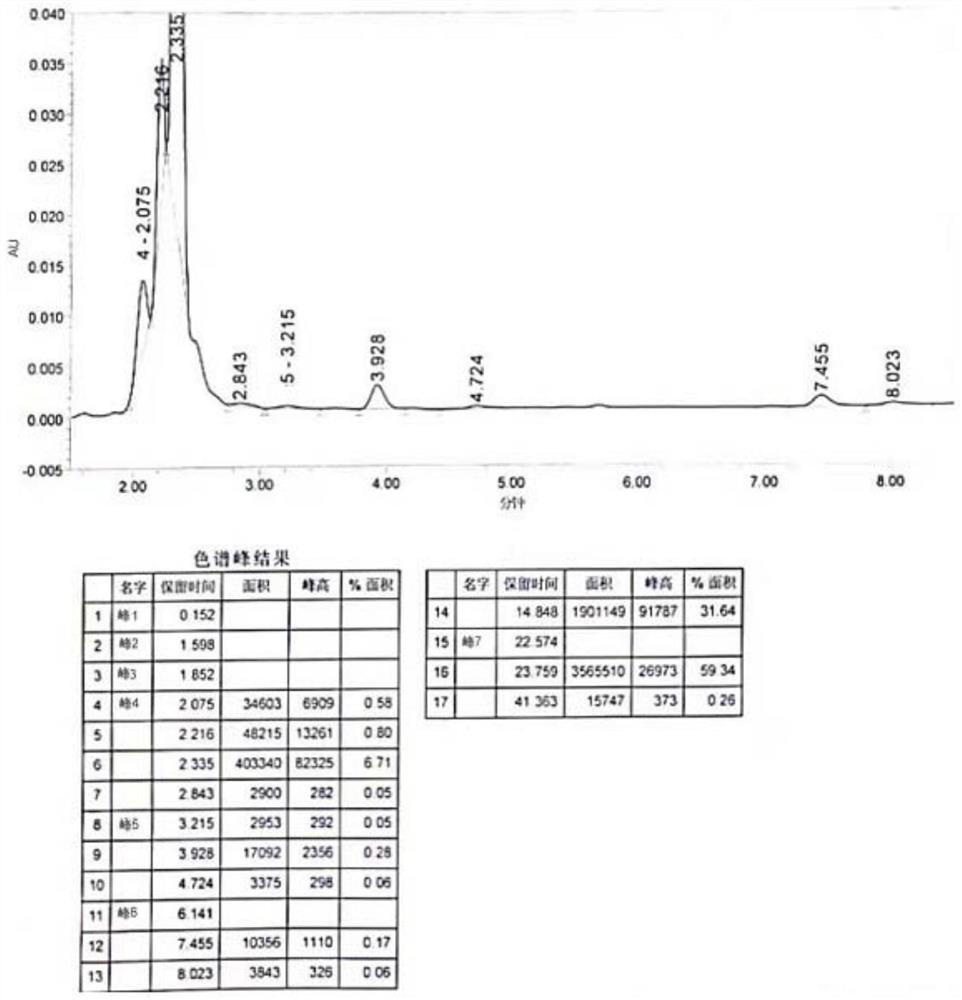
[0051] Add DMSO (100L), 3-(1-piperidinylmethyl)phenol (48kg), 3-chloropropylamine hydrochloride (40kg) into the reaction kettle, stir, add sodium hydroxide (140kg), and heat up to 90°C , and monitor the reaction until it stops. Filtrate, add water to the filtrate, stir for 15 min, extract twice with dichloromethane, wash the organic layer with saturated brine, add anhydrous sodium sulfate to dry, filter and evaporate the filtrate to give a light yellow oil 3-[3-(1-piperone Pyridylmethyl) phenoxyl group] alanine (54.2kg), about 87% yield, 99.45% purity (see Image 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com