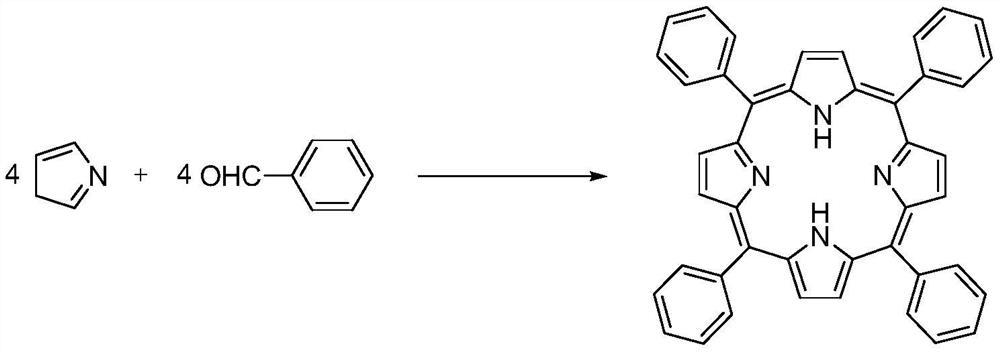
A method for synthesizing tetraphenylporphyrin in a reflux tubular reactor
A technology of tubular reactor and tetraphenylporphyrin, which is applied in the direction of organic chemistry, can solve the problems of large amount of catalyst usage, unhandy waste liquid, sticky acidic filtrate, etc., so as to avoid the process of raw material recovery and reduce the use of catalyst Quantity, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
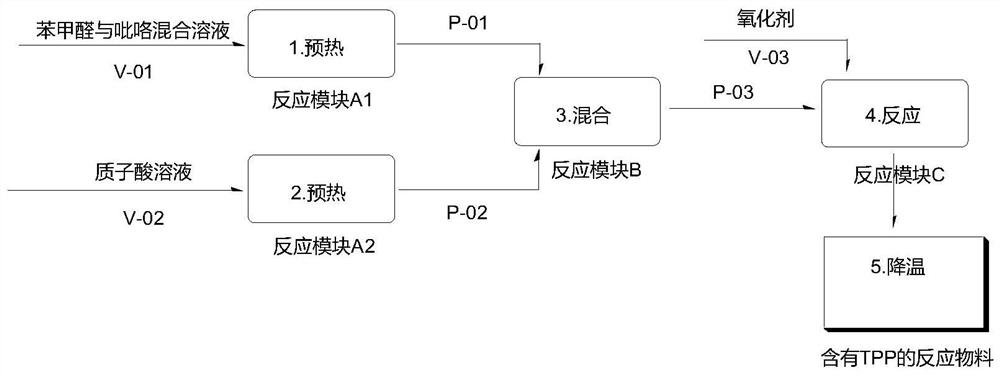
[0033] 1) After the two reaction modules A1 and A2 are connected in parallel with the two reaction modules B and C, the microchannel reactor is replaced with proton acid; benzaldehyde 106g (1mol) and pyrrole 67g (1mol) are mixed into a homogeneous solution and put into the storage tank V-01, the volume is about 170mL; the propionic acid 680g is put into the storage tank V-02, the volume is about 681mL;
[0034] 2) The volume flow rate of the mixed solution of controlled benzaldehyde and pyrrole is 2.0 mL / min preheated by pump P-01 into the first reaction module A1 at 110 °C; the volume flow rate of controlled propionic acid is 7.8 mL / min by pump P-02 into the second reaction module A2 at 120 °C; and then mixed in the third reaction module B At the same time that the mixed material enters the fourth reaction module C, the nitrobenzene 184.5g of the pre-storage tank V-03 is pumped into the pipeline; the delay pipeline of the tube reactor is completed in 3 minutes, and the reaction t...
Embodiment 2
[0039] 1) After the two reaction modules A1 and A2 are paralleled with the two reaction modules of B and C, the microchannel reactor is replaced with proton acid; the benzaldehyde 106g (1mol) and pyrrole 67g (1mol) are mixed into a homogeneous solution and put into the storage tank V-01, the volume is about 170mL; the acetic acid 727g is put into the storage tank V-02, the volume is about 693mL;
[0040] 2) The volume flow rate of the mixed solution of benzaldehyde and pyrrole is controlled by 2.0 mL / min by pump P-01 into the first reaction module A1 to preheat 120 °C; the volume flow rate of controlling acetic acid is 8.2 mL / min by pump P-02 into the second reaction module A2 to preheat 115 °C; and then into the third reaction module B to mix; the compressed air is passed into 80 cm3 / min while the mixed material enters the fourth reaction module C; the delay line of the warp tube reactor, The time is completed in 10min, and the reaction temperature is 125 °C;
[0041] 3) The mate...
Embodiment 3
[0045] 1) After the two reaction modules A1 and A2 are paralleled with the two reaction modules of B and C, the microchannel reactor is replaced with proton acid; the benzaldehyde 106g (1mol) and pyrrole 67g (1mol) are mixed into a homogeneous solution and put into the storage tank V-01, the volume is about 170mL; the acetic anhydride 788g is put into the storage tank V-02, the volume is about 730mL;
[0046] 2) The volume flow rate of the mixed solution of benzaldehyde and pyrrole is controlled by 2.0 mL / min by pump P-01 into the first reaction module A1 to preheat 120 °C; the volume flow rate of controlling acetic anhydride is 9.2 mL / min by pump P-02 into the second reaction module A2 to preheat 115 °C; and then into the third reaction module B to mix; the compressed air is compressed to pass into 30 cm3 / min at the same time as the mixed material enters the fourth reaction module C; the trained tube reactor delays the pipeline. The time is 7min to complete, and the reaction temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com