Synthesis method of osimertinib intermediate
A synthetic method and intermediate technology, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of expensive raw materials, difficult to obtain in the market, complicated process, etc., and achieve the effects of short reaction time, good impurity removal effect, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
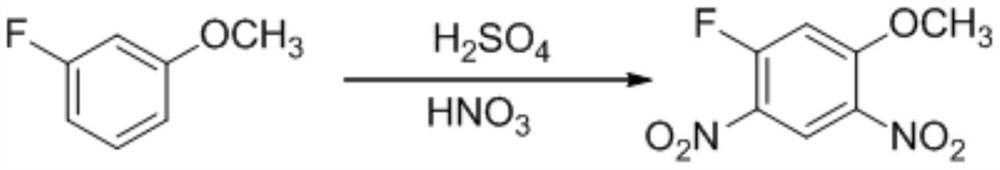
[0046] 1) Preparation of 3-fluoro-4,6-dinitroanisole (intermediate 1)
[0047] In a 1L reaction flask, add 792g of 98% concentrated sulfuric acid, add 160g of 3-fluoroanisole in batches under stirring, after stirring evenly, cool down to -10℃~-5℃, control the temperature -10~0℃ and add concentrated Nitric acid (98%) 183g, after dropwise addition, stir for 10 minutes, heat up to normal temperature 20-25°C, and stir for 3 hours. Spot the plate (developing agent: ethyl acetate: petroleum ether = 2:1), after the reaction is completed, slowly drop the system into 1500mL ice water, control the temperature below 20°C, after the dropwise addition, control the temperature at 30-35°C and stir for 3 hours . Filter, rinse the filter cake with a small amount of water, and dry to obtain 246.9 g of a khaki product. The purity is 98.5%, and the yield is 90%.
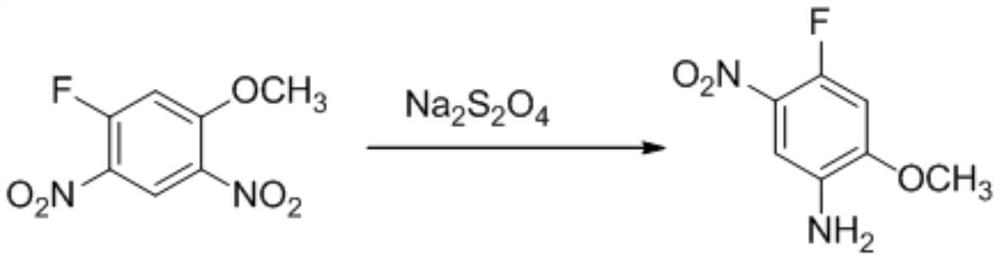
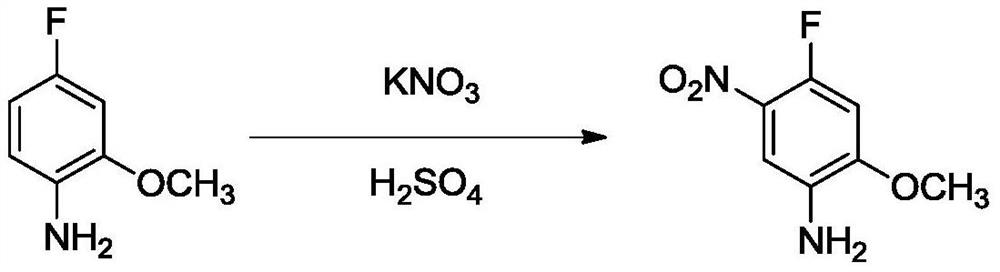
[0048] 2) Preparation of 4-fluoro-2-methoxy-5-nitroaniline
[0049] In a 1L reaction flask, add 300mL of water, add 60g of 3-fluor...
Embodiment 2
[0051] 1) Preparation of 3-fluoro-4,6-dinitroanisole (intermediate 1)
[0052]In a 1L reaction flask, add 792g of 98% concentrated sulfuric acid, add 160g of 3-fluoroanisole in batches under stirring, after stirring evenly, cool down to -10℃~-5℃, control the temperature -10~0℃ and add concentrated Nitric acid (95%) 210g, after the dropwise addition, stir for 10 minutes, heat up to normal temperature 20-25°C, and stir for 3 hours. Spot the plate (developing agent: ethyl acetate: petroleum ether = 2:1), after the reaction is completed, slowly drop the system into 1500mL ice water, control the temperature below 20°C, after the dropwise addition, control the temperature at 30-35°C and stir for 3 hours . After filtering, the filter cake was rinsed with a small amount of water, and dried to obtain 230 g of a khaki product. The purity is 97.8%, and the yield is 83.8%.
[0053] 2) Preparation of 4-fluoro-2-methoxy-5-nitroaniline
[0054] In a 1L reaction flask, add 300mL of water,...
Embodiment 3
[0056] 1) Preparation of 3-fluoro-4,6-dinitroanisole (intermediate 1)
[0057] In a 1L reaction flask, add 900g of 95% concentrated sulfuric acid, add 160g of 3-fluoroanisole in batches under stirring, after stirring evenly, cool down to -10℃~-5℃, control the temperature -10~0℃ and add concentrated Nitric acid (65%) 280g, after the dropwise addition, stir for 10 minutes, heat up to normal temperature 20-25°C, and stir for 3 hours. Spot the plate (developing agent: ethyl acetate: petroleum ether = 2:1), after the reaction is completed, slowly drop the system into 1500mL ice water, control the temperature below 20°C, after the dropwise addition, control the temperature at 30-35°C and stir for 3 hours . After filtration, 216 g of a khaki solid was obtained, with a purity of 97.5% and a yield of 78.7%.
[0058] 2) Preparation of 4-fluoro-2-methoxy-5-nitroaniline
[0059] In a 1L reaction flask, add 360mL of water, add 60g of 3-fluoro-4,6-dinitroanisole prepared above under stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com