Development of hMSC production kit and optimization of quality management standards and establishment of quality management system
A kit and standard technology, applied in the field of stem cell therapy, can solve problems such as the lack of research on cell quality stability, the establishment of cell quality evaluation and subject evaluation systems, and the difficulty of forming quality standards, so as to optimize primary tissue processing Improvement of technology, yield of primary cells, reduction of raw material consumption and production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 Study on the quality of industrialized production process of human umbilical cord MSC
[0063] 1. Cell Identification
[0064] (1) Cell morphology:
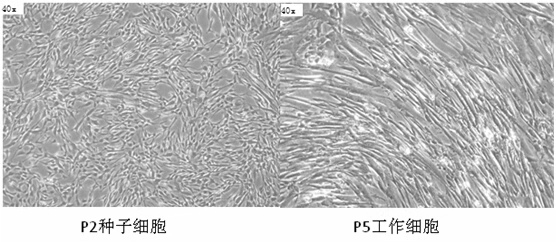
[0065] It is generally believed that under standard culture conditions, the morphology of human umbilical cord mesenchymal stem cells is fibroblast-like or spindle-shaped, and grows adherently. Under a high-power microscope, the cells should have a strong three-dimensional sense and fewer intracellular granules. See figure 1 .
[0066] (2) Cytogenetic identification:
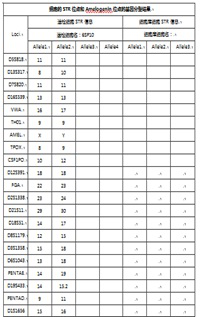
[0067] The source of human umbilical cord mesenchymal stem cells should be single to ensure the stability and controllability of cell function. When determining the source, use STR map for genetic analysis to confirm that the source of the cells is a single source of cell donors. See figure 2 ;
[0068] STR profile analysis shows that after the isolated cells are amplified on 21 autosomal allele loci, the test results are all single-peak or...
Embodiment 2
[0136] Preparation and use of the culture kit of embodiment 2 hMSC
[0137] The present invention provides a kind of MSC induction culture kit, comprises container and the clinical MSC cell serum-free expansion medium MSC- and cell culture supplement MSC- :
[0138] 1) The present invention provides a clinical MSC cell expansion complete culture medium MSC- , PH7.2~7.4, balanced salt Earle's salt, nucleoside, L-glutamine 2mM, NaHCO3 2200mg / L, D-glucose 1000mg / L, sodium pyruvate 1mM, phenol red indicator 10mg / L, inorganic salt , amino acids, vitamins, ribonucleosides, deoxyribonucleosides, etc., add 2mM GlutaMAX™-I and 10% Prime serum during use, and the present invention does not exclude the use of medium other than the above-mentioned medium for cultivation;
[0139] 2) The present invention provides a MSC cell culture additive MSC- , which contains 2-50ng / mL bFGF, 2-50ng / ml EGF;
[0140] The preparation of described complete medium: MSC- Add 1 / 1000 MSC- , 2mM Gl...
Embodiment 3
[0145] Example 3 hMSC cell culture supplement MSC- The formula is further optimized
[0146] The experiment is divided into 6 groups:
[0147] Group A: α-MEM +3ng / mL bFGF+3ng / ml EGF+10%FBS+Gln;
[0148] Group B: α-MEM+6ng / mL bFGF+6ng / ml EGF+10%FBS+Gln;
[0149] Group C: α-MEM+6ng / mL bFGF+6ng / ml EGF+6ng / ml VEGF+6ng / ml PDGFP+10%FBS+Gln;
[0150] Group D: α-MEM +3ng / mL bFGF+3ng / ml EGF+3ng / ml VEGF+3ng / ml PDGF+10%FBS+Gln;
[0151] Group E: α-MEM +3ng / mL bFGF+3ng / ml EGF+6ng / ml VEGF+6ng / ml PDGF+10%FBS+Gln;
[0152] Prime serum group: α-MEM +6ng / mL bFGF+6ng / ml EGF+56ml Prime+5ml Gln
[0153]Under the six groups of culture conditions, the umbilical cord MSCs were continuously cultured to the P3 generation, and the growth curve was drawn in Figure 5 :
[0154] Statistical analysis of six groups of data, there was no significant difference between groups, P=0.62, P>0.05;
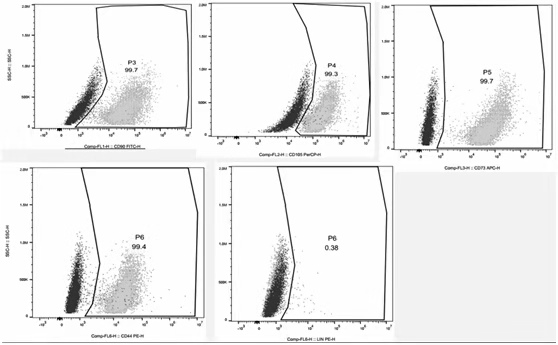
[0155] The results of flow cytometry detection of umbilical cord MSC cultured under six groups of culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com