Tri-rare-earth fluorescence sensor capable of being used for recognizing human metabolites
A technology of trimesic acid and europium terbium, which is applied in the field of preparation of lanthanum trimesate, can solve the problem of fewer fluorescent sensors for uric acid and achieve accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] refer to Figure 13 , the preparation and application of three rare earth fluorescent sensors that can be used to identify human metabolites, the steps are as follows:
[0033] S1, select raw materials, lanthanum nitrate, terbium nitrate, europium nitrate and trimesic acid;
[0034] S2, dissolving trimesic acid in ethanol, stirring;
[0035] S3, first adding lanthanum nitrate solution to the solution obtained in S2, then adding different proportions of terbium nitrate and europium nitrate solutions, and stirring;
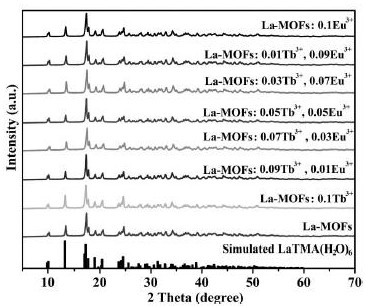
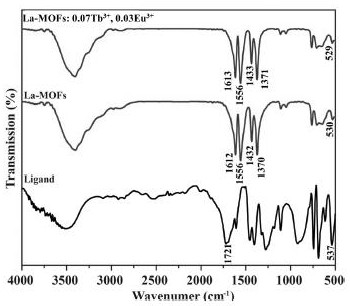
[0036] S4, standing still, centrifugal washing, drying to obtain a white sample;
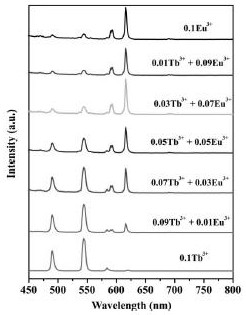
[0037] S5, characterizing the obtained lanthanum trimesate materials with different doping amounts of europium and terbium;
[0038] S6, the use of europium-terbium-doped lanthanum trimesate for the detection of human metabolite uric acid.
[0039] Further, in both step S2 and step S3, stir at room temperature for 30 min.
[0040] Further, add europium ion mole fraction in...
Embodiment 2
[0067] The lanthanum trimesate material doped with europium and terbium of the present invention is used as a fluorescent probe to detect uric acid in urine.
[0068] Water Stability:
[0069] Such as Figure 5 Shown are the XRD pattern and the fluorescence emission spectrum pattern of the preferred sample immersed in water for different times. It is preferred that the sample has good pH stability after two weeks storage in water.
[0070] Such as Figure 6 Shown are the XRD patterns and fluorescence emission spectra of preferred samples immersed in different pH solutions. The preferred samples also exhibit good pH-independent luminescence and structural stability in the pH range of 3-10, indicating that the preferred samples have good compatibility with the aqueous environment. These results indicate that the preferred samples are promising for fluorescent sensors to detect specific human urine metabolites.
[0071] Optional test:
[0072] Figure 7 The effect of the v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com