Creatine kinase isoenzyme (CK-MB) detection kit capable of resisting heparin interference
A detection kit, creatine kinase technology, which can be used in measurement devices, instruments, scientific instruments, etc., can solve the problem of high measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
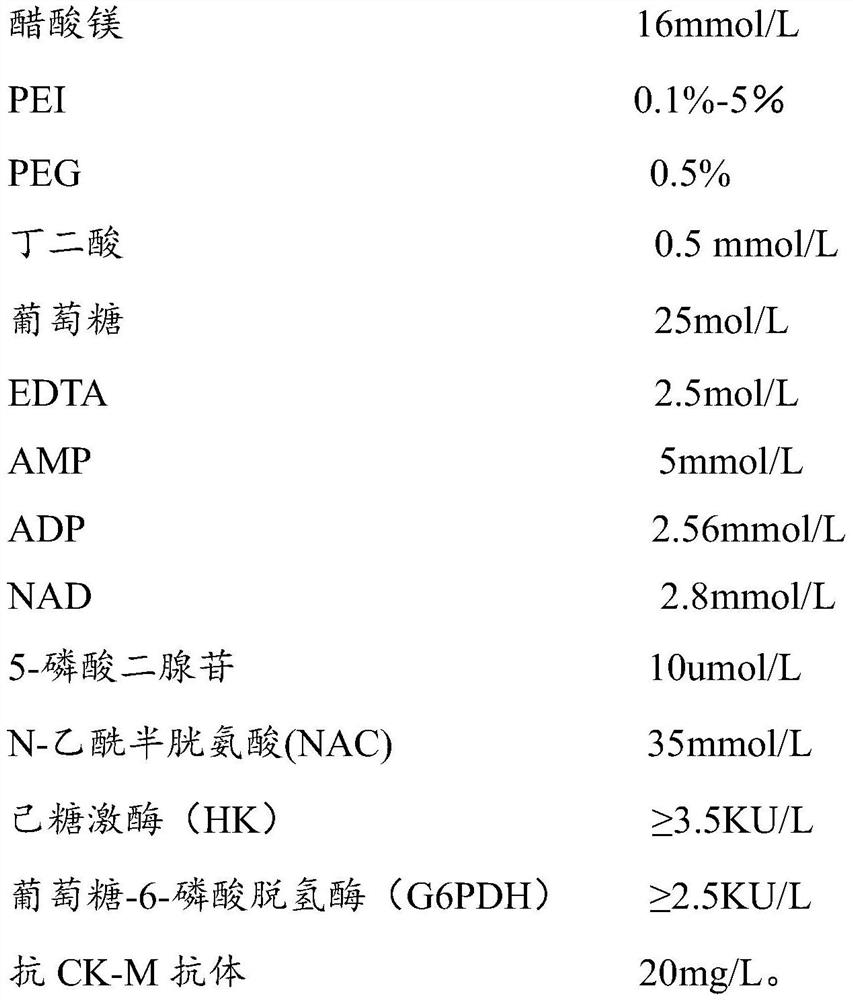
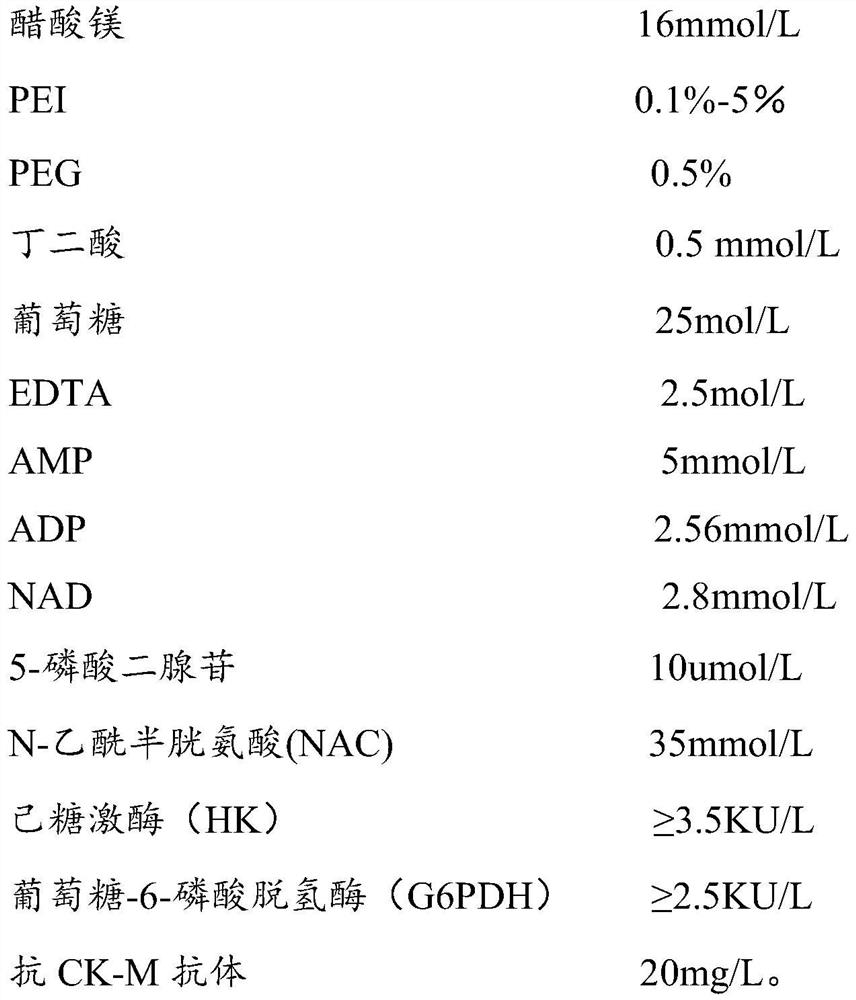
[0035] The kit of the present invention comprises R1 reagent and R2 reagent, and described R1 reagent comprises: Magnesium acetate, polyethyleneimine (PEI), PGE, succinic acid, glucose, EDTA, AMP, ADP, NAD, 5-diadenosine phosphate , N-acetylcysteine, hexokinase, glucose-6-phosphate dehydrogenase and anti-CK-MB antibody, the R2 reagent includes: phosphocreatine.
[0036] The concentration of each substance in the R1 reagent is as follows:
[0037]
[0038] The concentration of each substance in the R2 reagent is as follows:
[0039] Imidazole buffer 63.5mmol / L
[0040] Phosphocreatine 4.3g / L
[0041] Parabens 13%.
[0042] The volume ratio of R1 reagent to R2 reagent is 3:1.
Embodiment 2
[0044] The steps of using the kit of Example 1 of the present invention to detect creatinine in a sample are as follows:
[0045] In the detection process, take the automatic Hitachi biochemical analyzer 7100 as an example. The main wavelength is 340nm (or near); the secondary wavelength is 410nm (or near); the light path of the cuvette is 1.0cm; the two-point velocity method; the rising reaction.
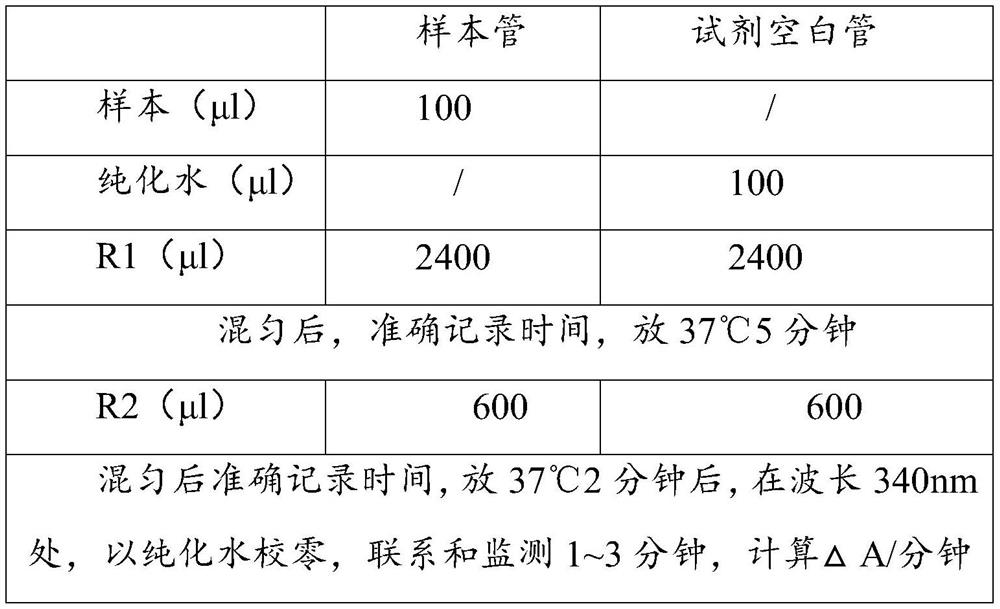
[0046] See Table 2 for reagent addition ratio:
[0047] Table 2 Reagent loading ratio
[0048]
[0049] The default unit of this kit is: U / L.
[0050] Calibration curve drawing: using Linear 2-point calibration curve calibration method. The value of the blank sample is 0.00 μmol / L, and the corresponding △A is measured for the calibrator, with △A as the Y axis and the concentration as the X axis. On the automatic analyzer, use Linear 2 points to establish the calibration curve. When calculating, find the corresponding concentration on the calibration curve according to the △...
Embodiment 3
[0054] In order to verify the probability of false positive detection between the invented kit and the commercially available creatine kinase assay kit, the best method is to use a third-party kit for verification, and the selected third-party kit is the Roche creatine kinase assay reagent box.
[0055] Among them, the three kits are: commercially available creatine kinase assay kit (kit I) without PEI, Roche creatine kinase kit (kit II) and the creatine kinase kit added in this embodiment. (Kit III). The reference range of the Roche creatine kinase kit is <25U / L, the reference range of the commercially available creatine kinase kit is <25U / L, and the reference range of the kit in this embodiment is <25U / L.
[0056] Source of specimens: 50 males with routine physical examination were selected as the research subjects. 5ml of venous blood was collected under fasting state and added to heparin anticoagulant blood vessels. The samples were centrifuged at low temperature for 5 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com