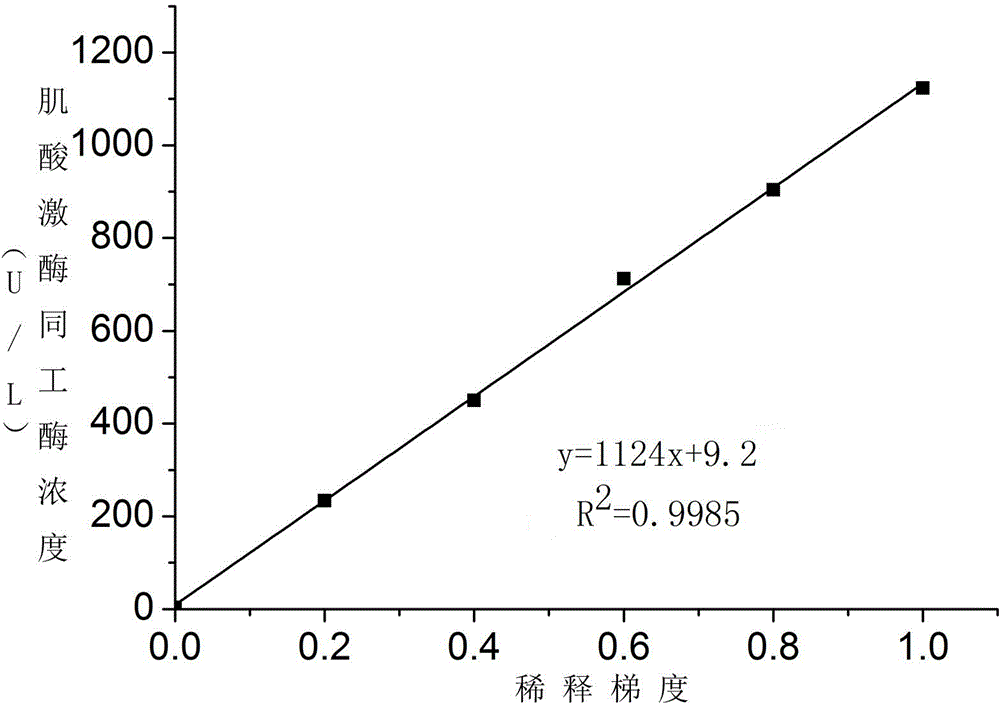
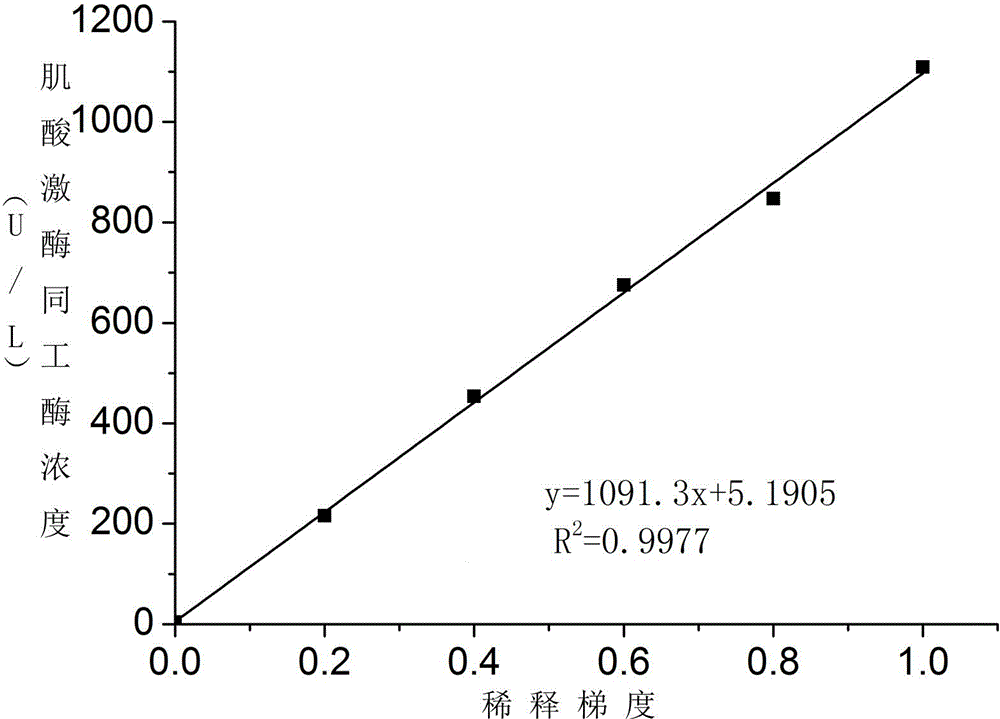
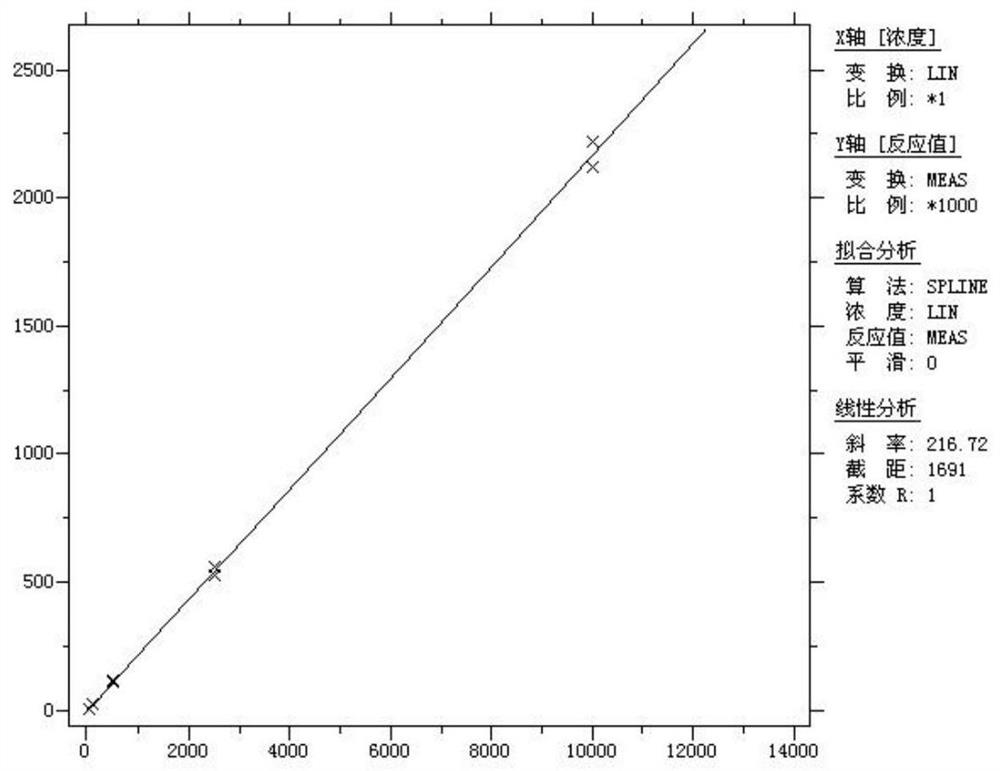
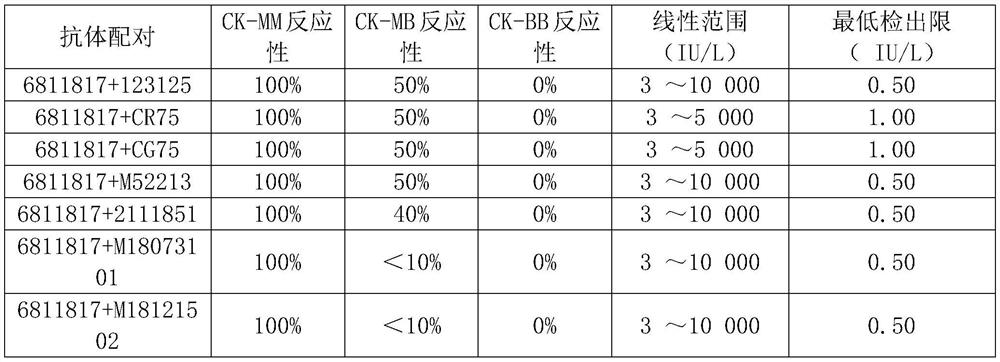
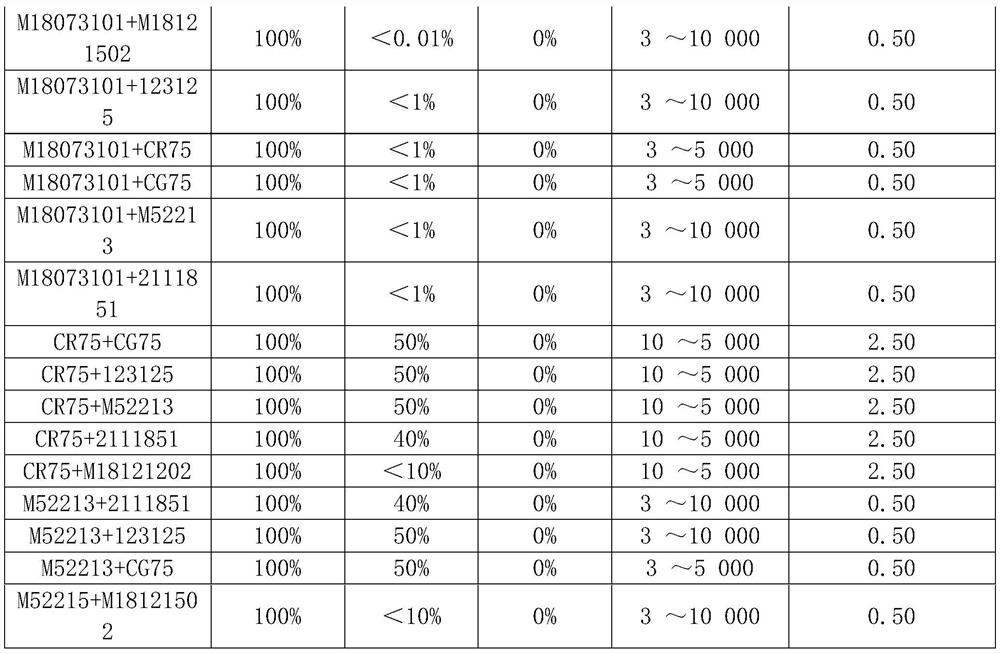
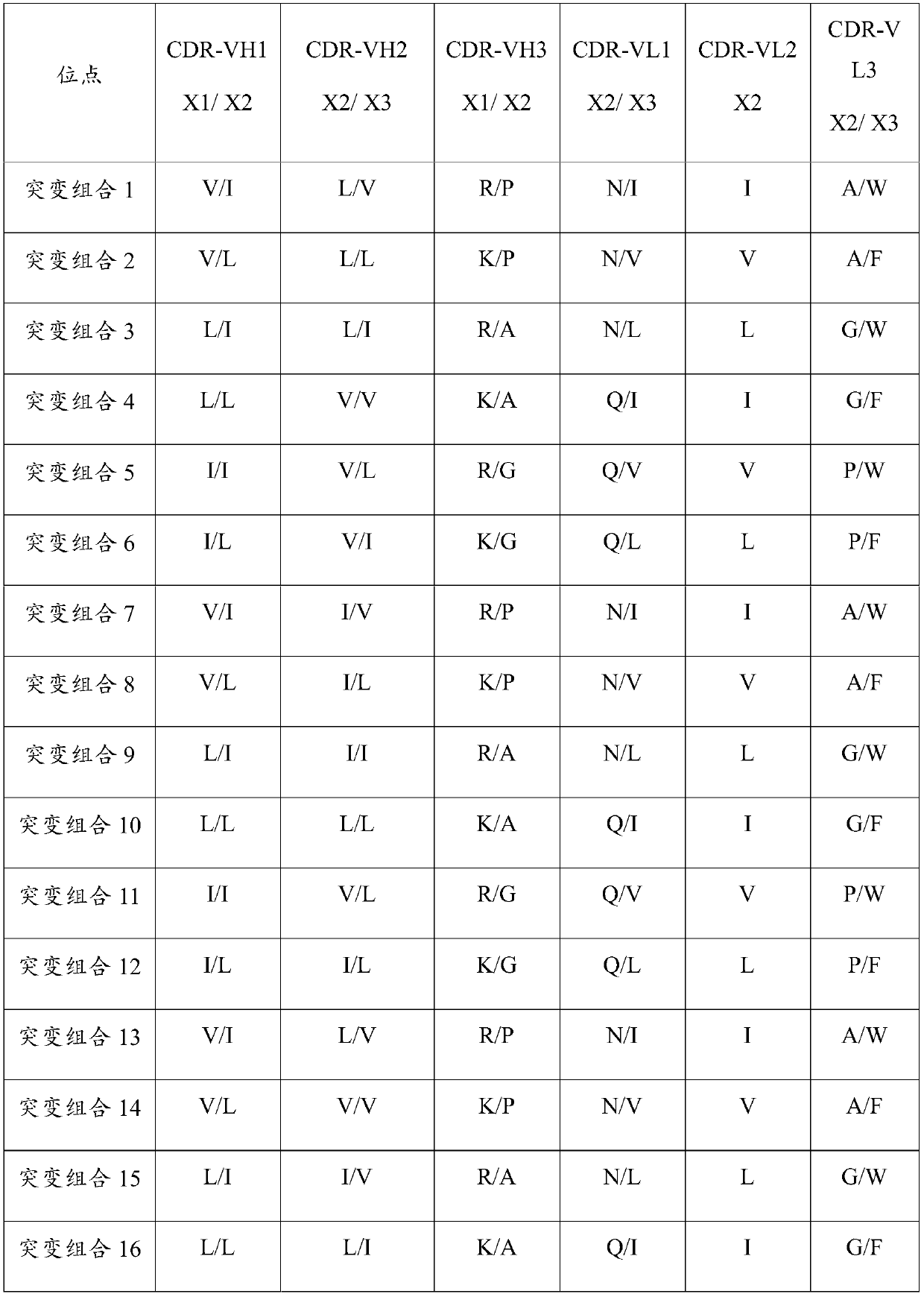
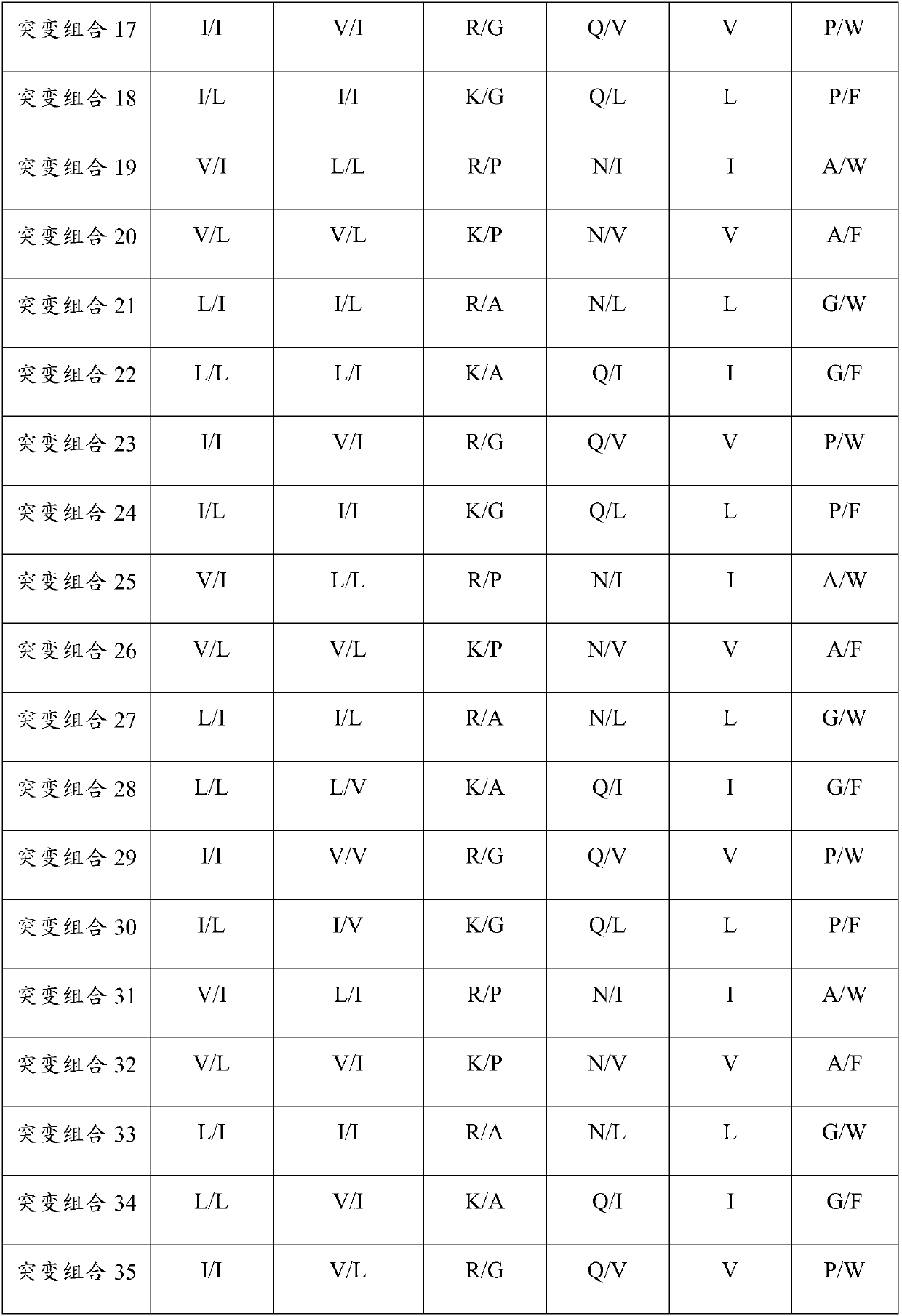
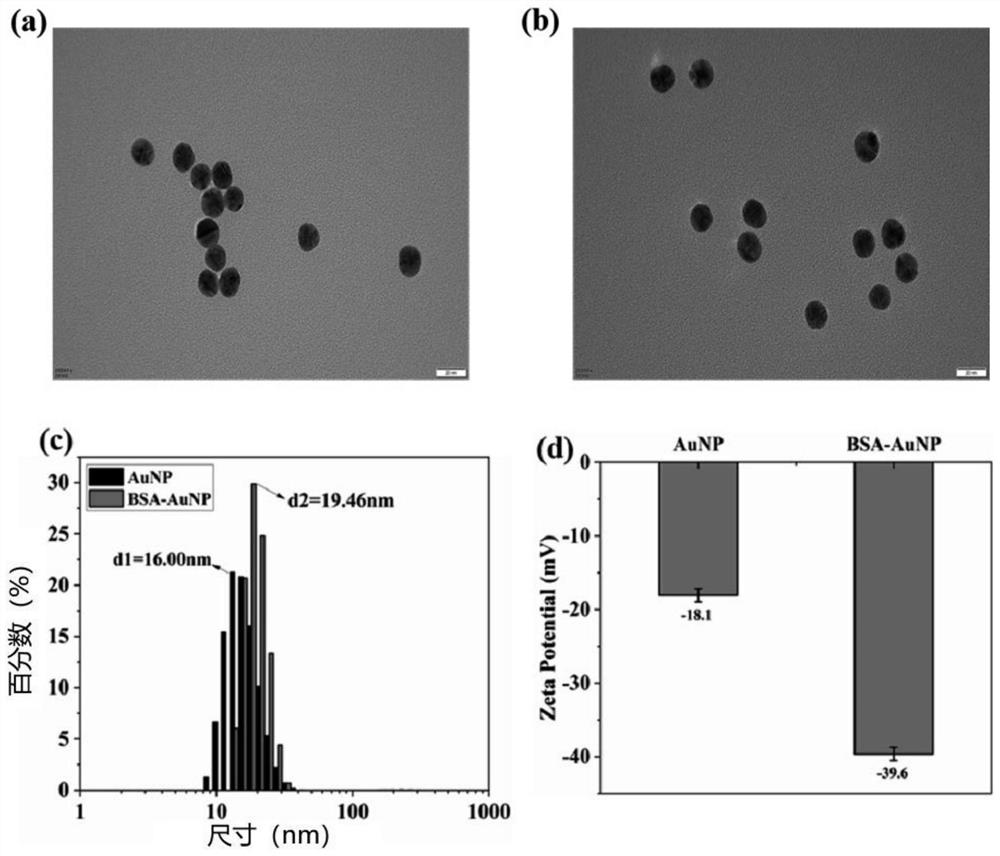
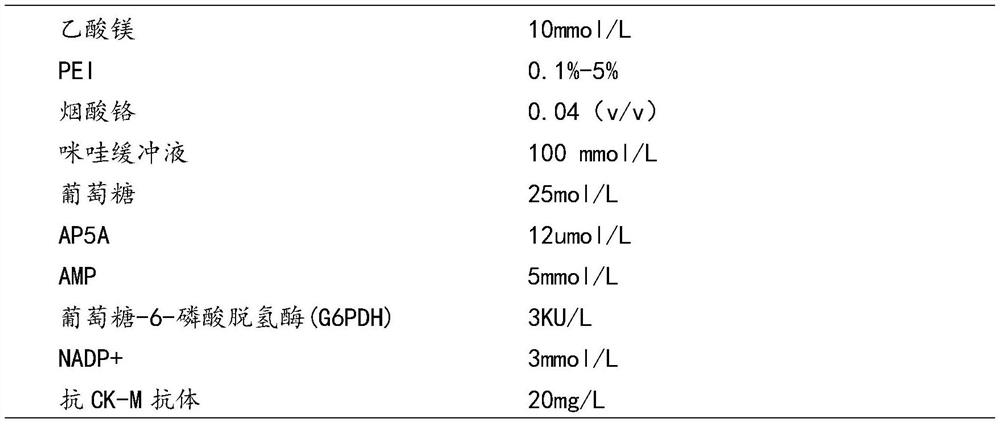
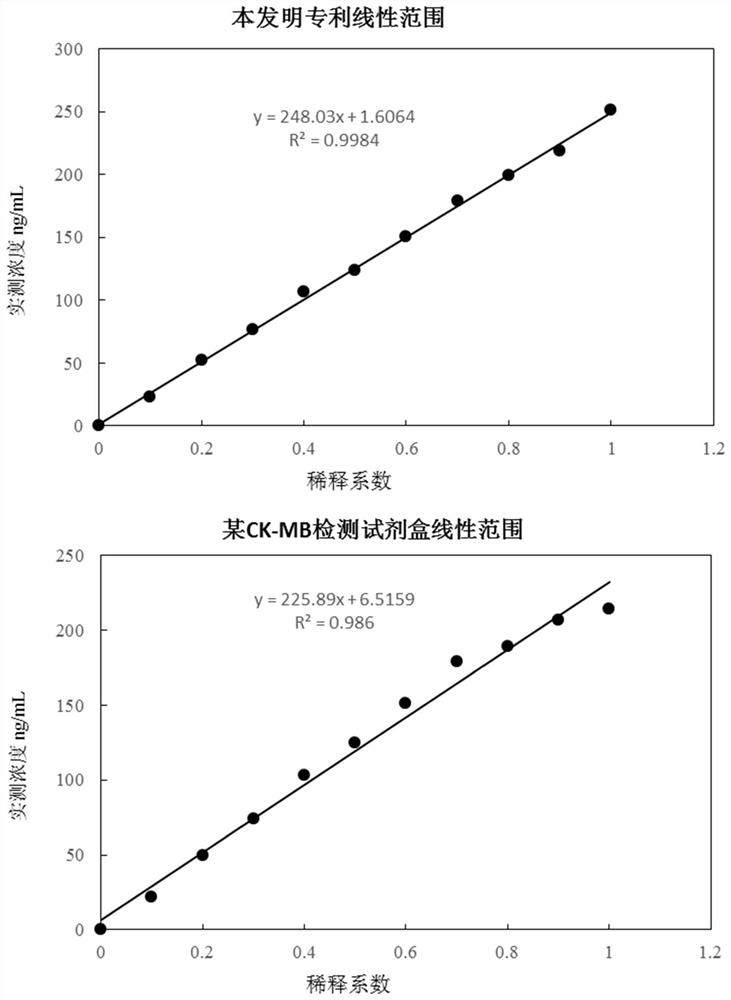
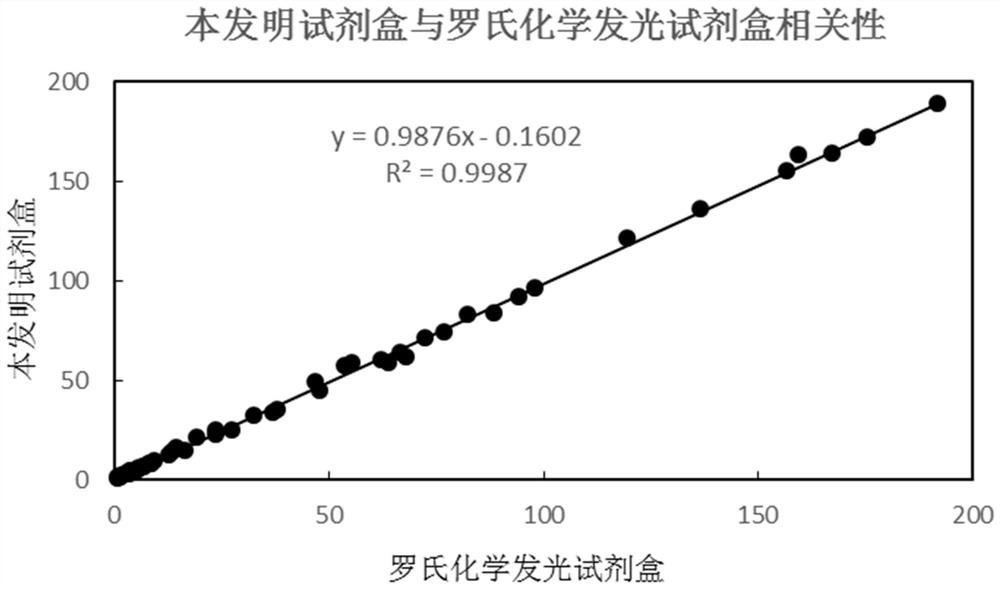
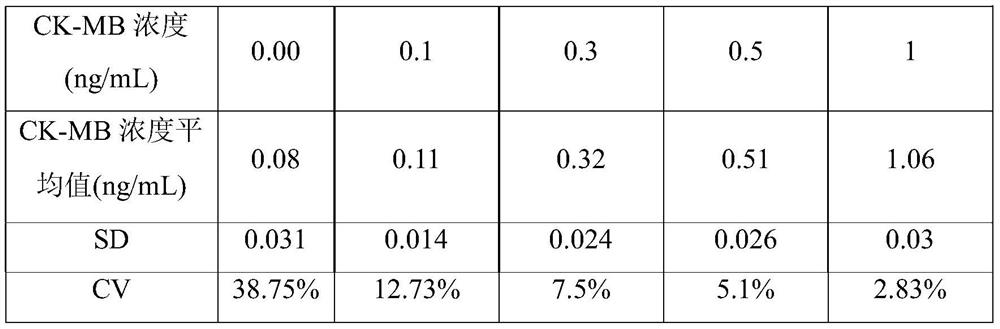
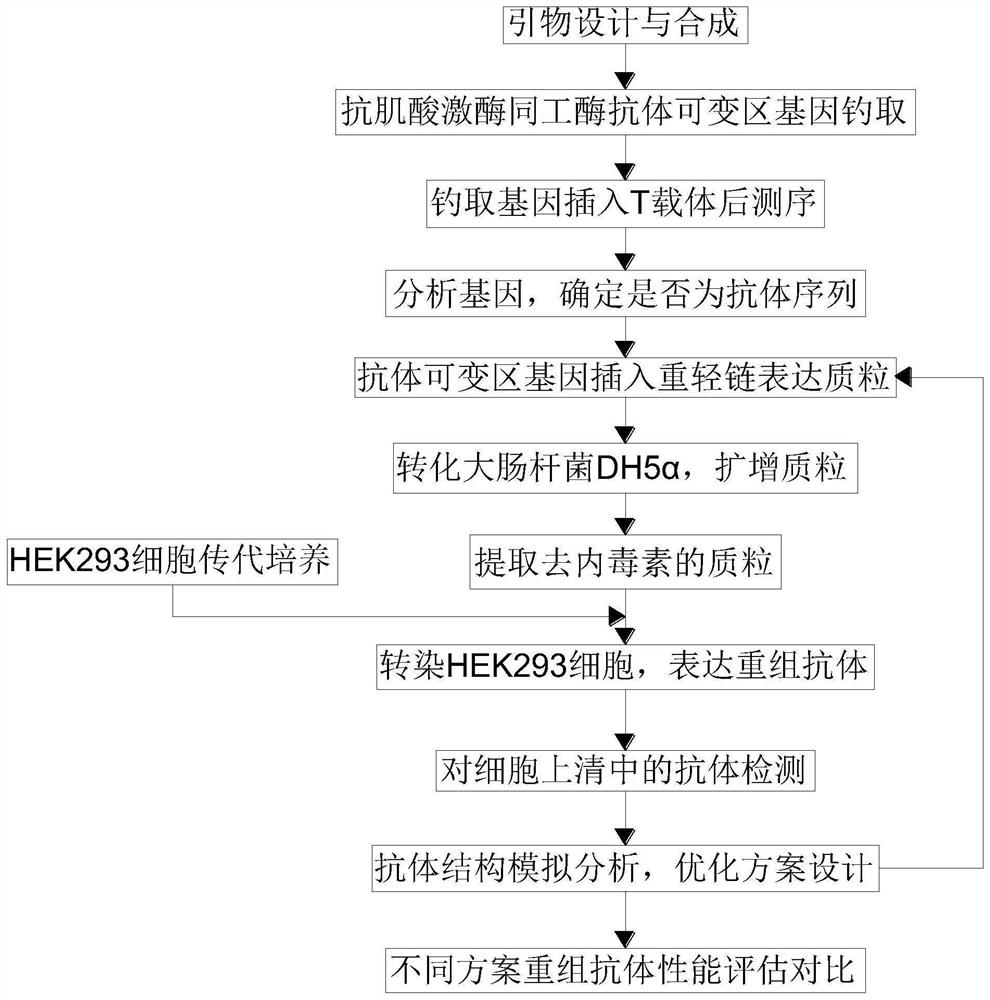
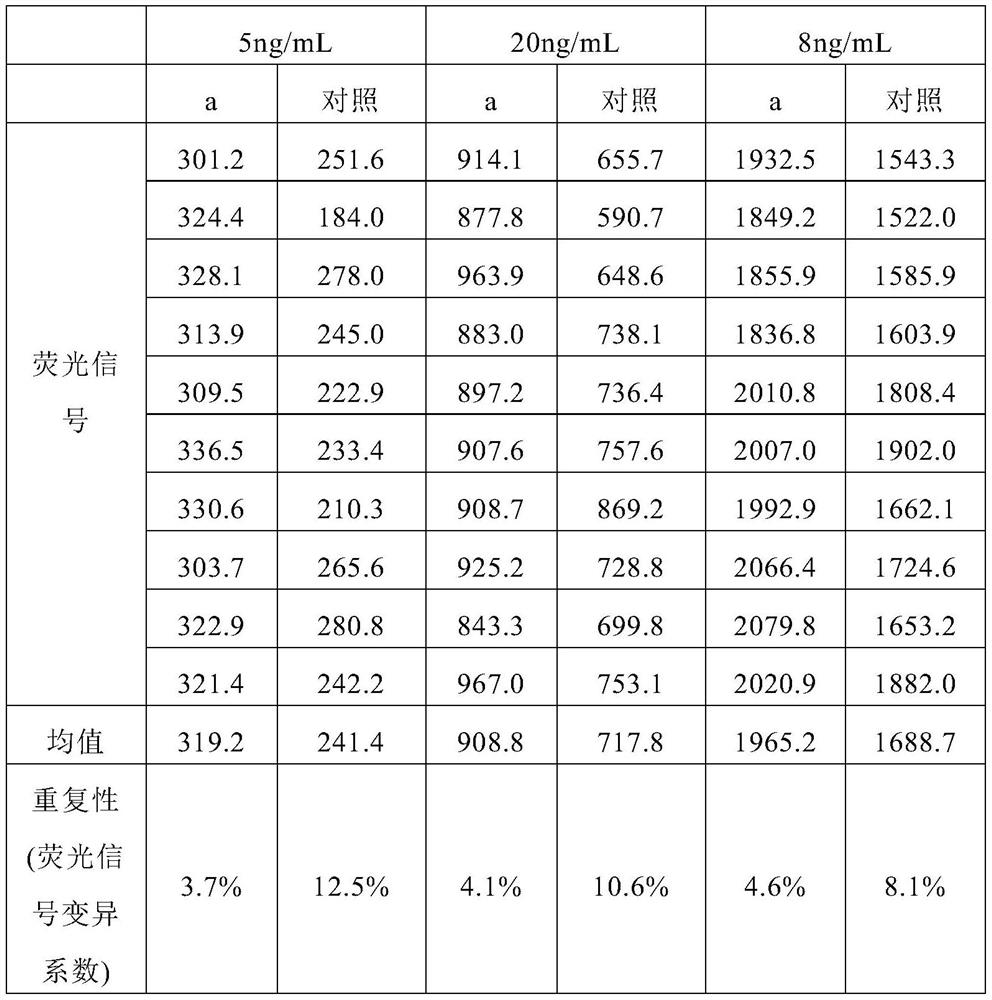
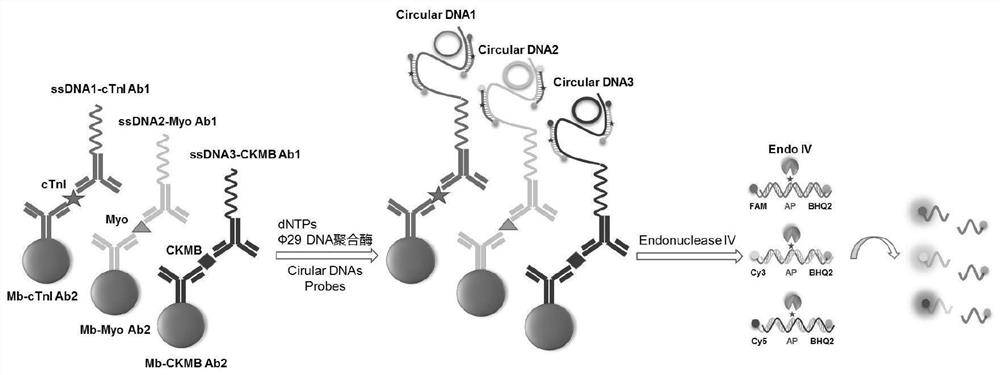
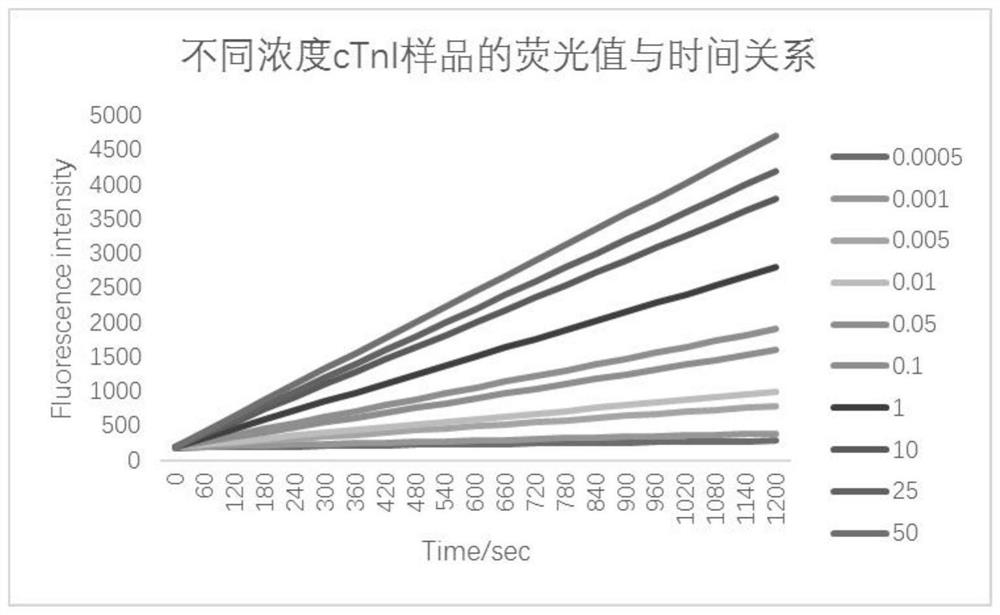
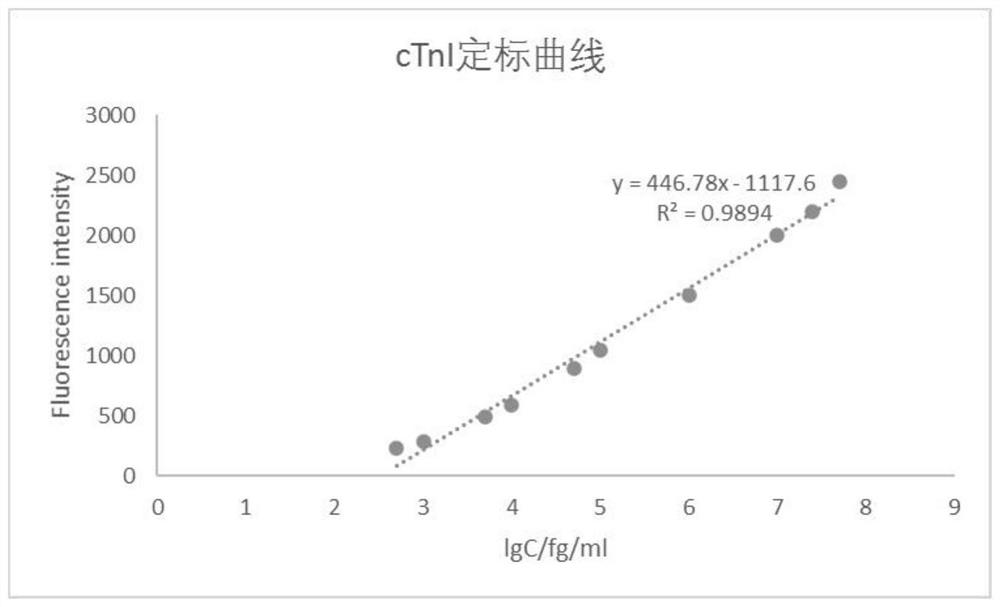
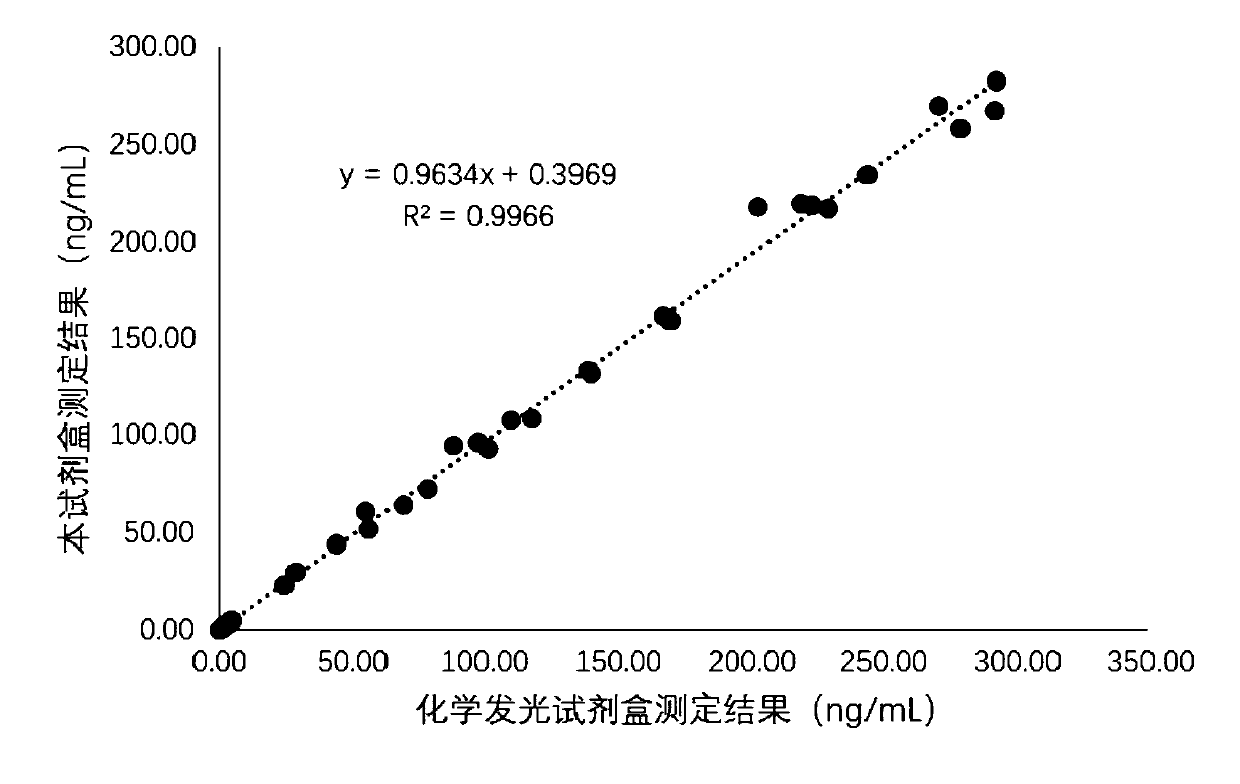
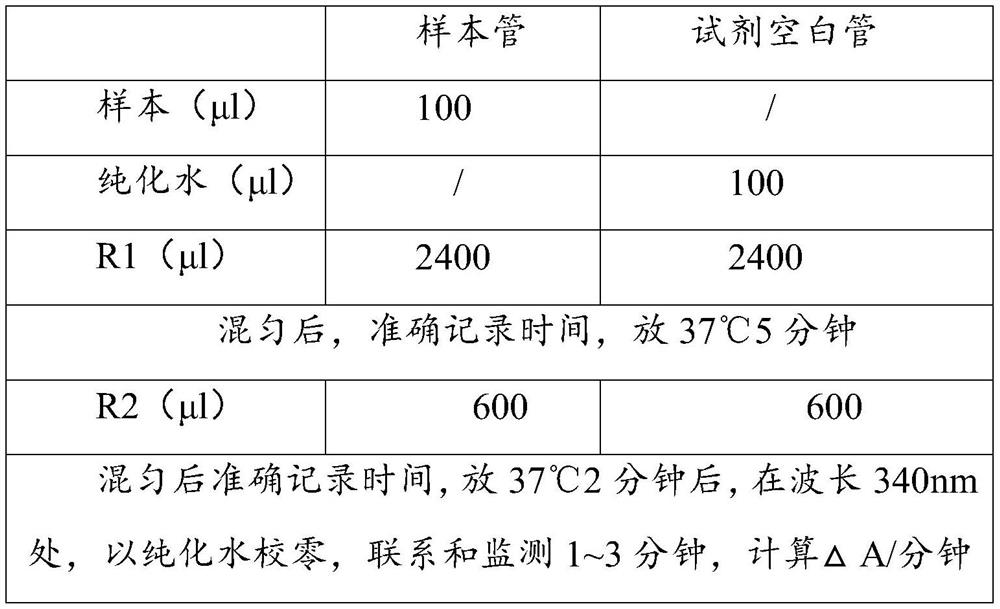
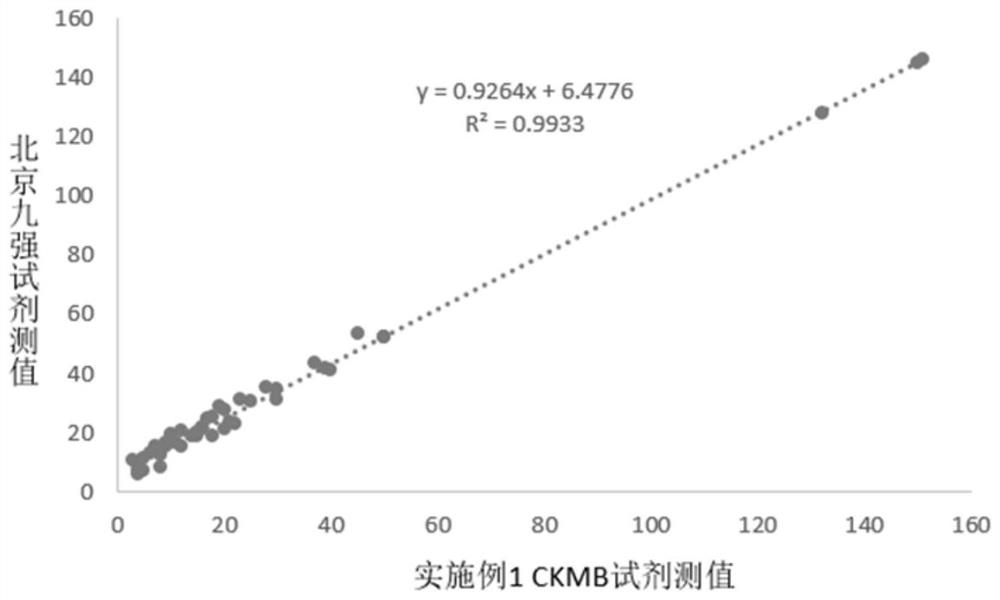
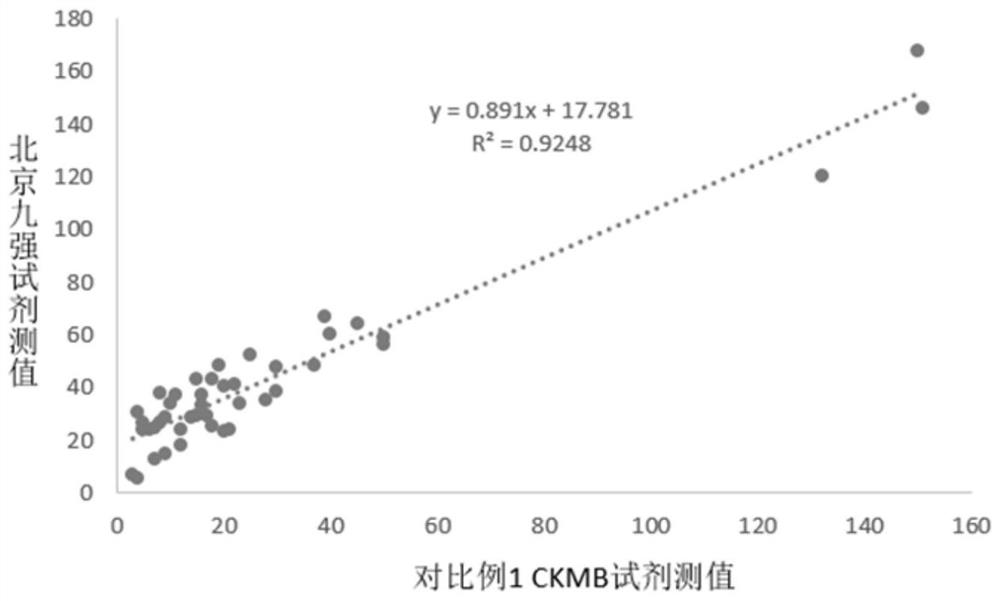
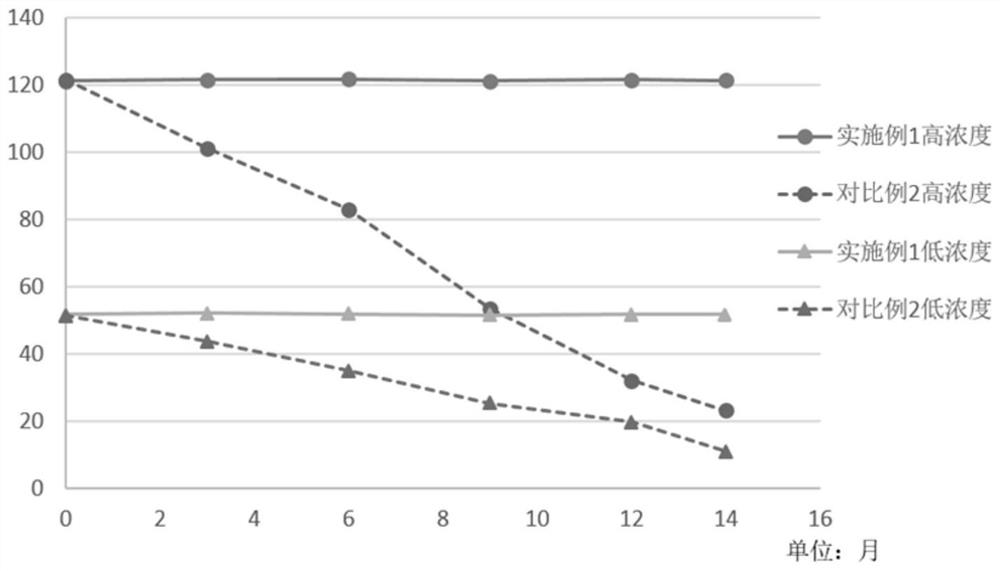
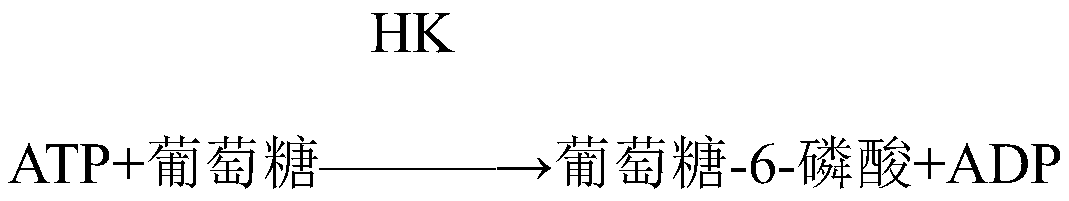
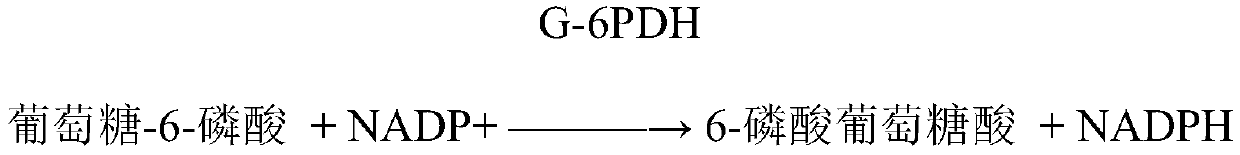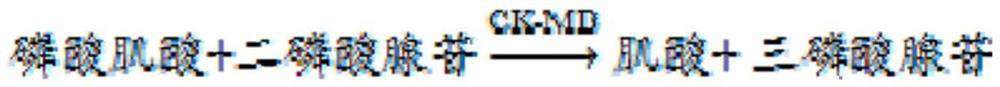Patents
Literature
39 results about "Creatine kinase isoenzymes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
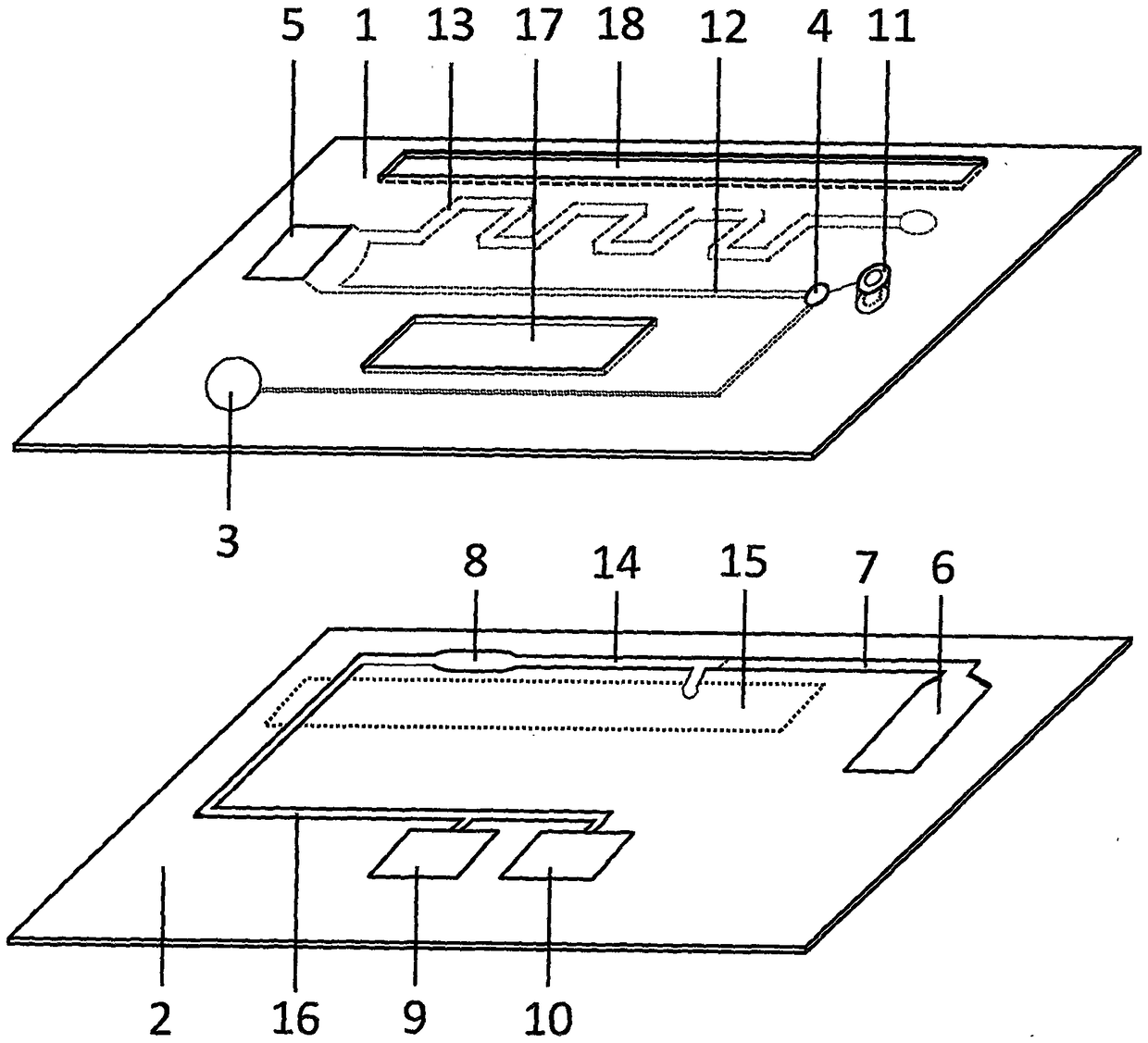
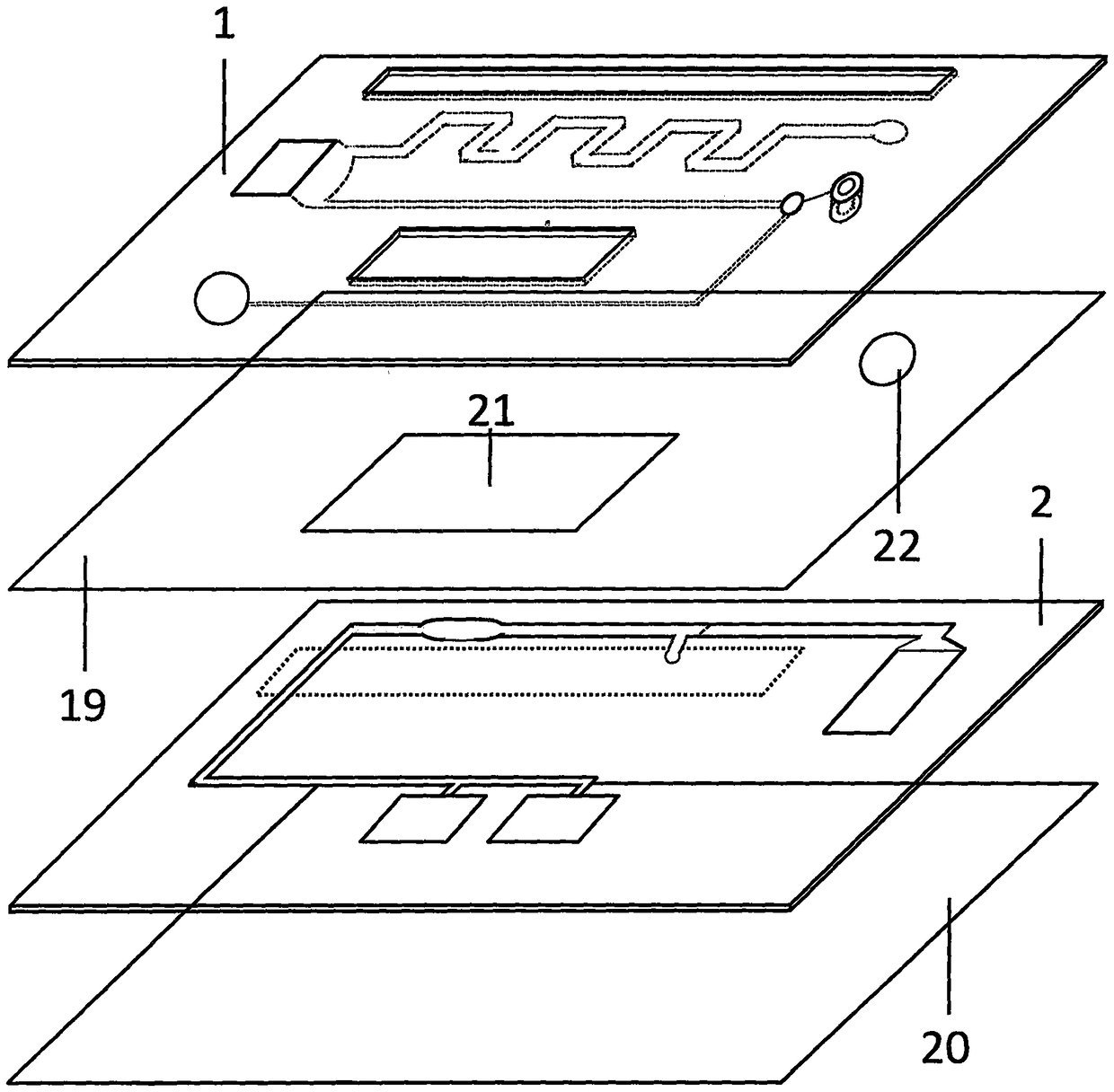
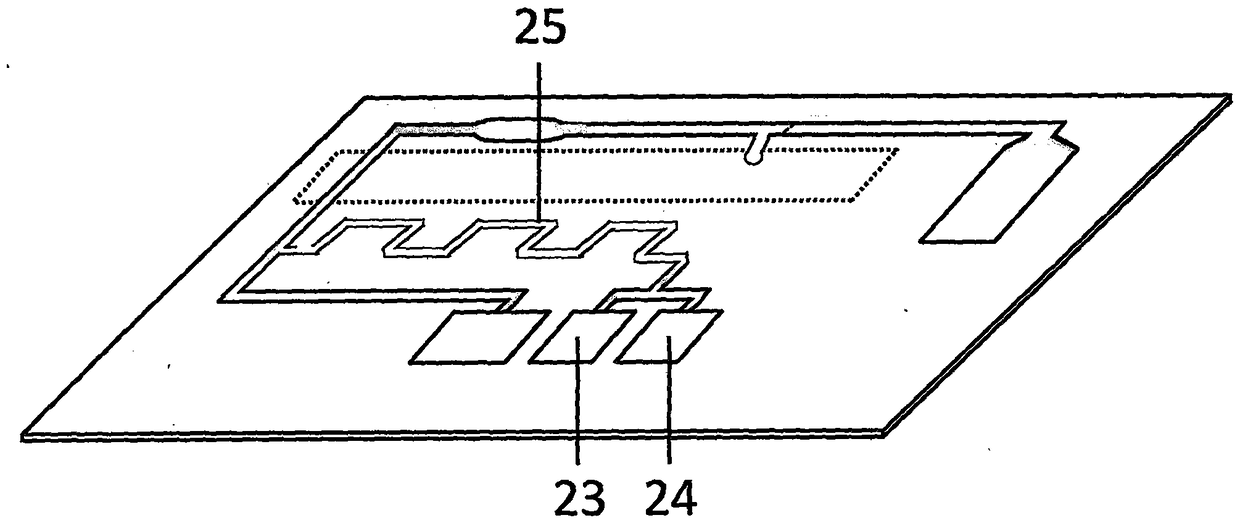
Test paper used for detecting acute myocardial infarction, and preparation method and application method thereof
InactiveCN102901828AHigh sensitivityRealize specific quantitative detectionBiological testingSerum igeCreatine kinase
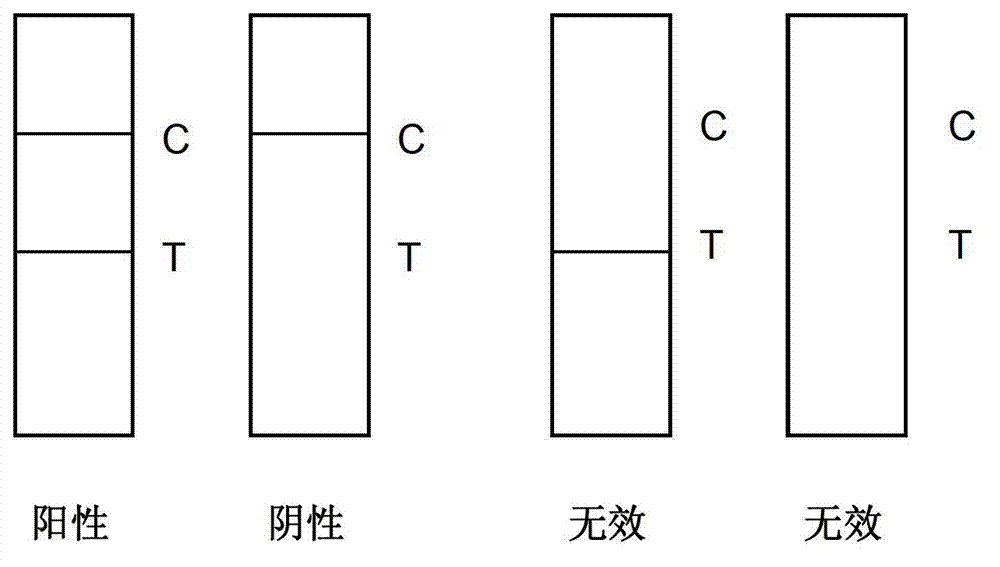
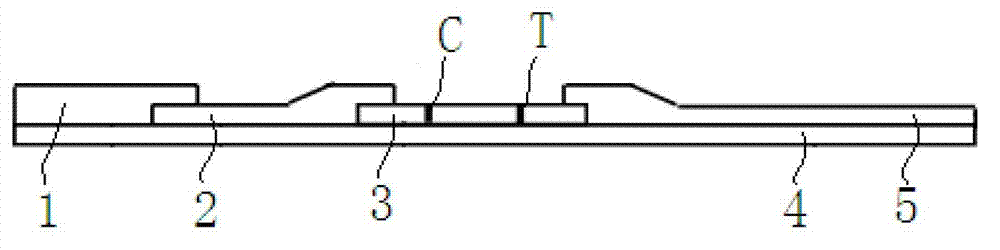
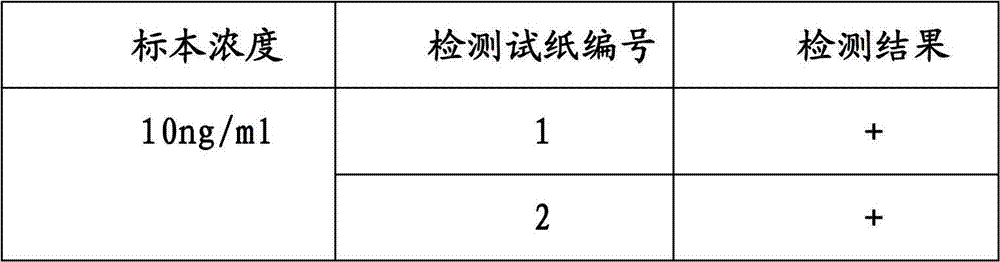
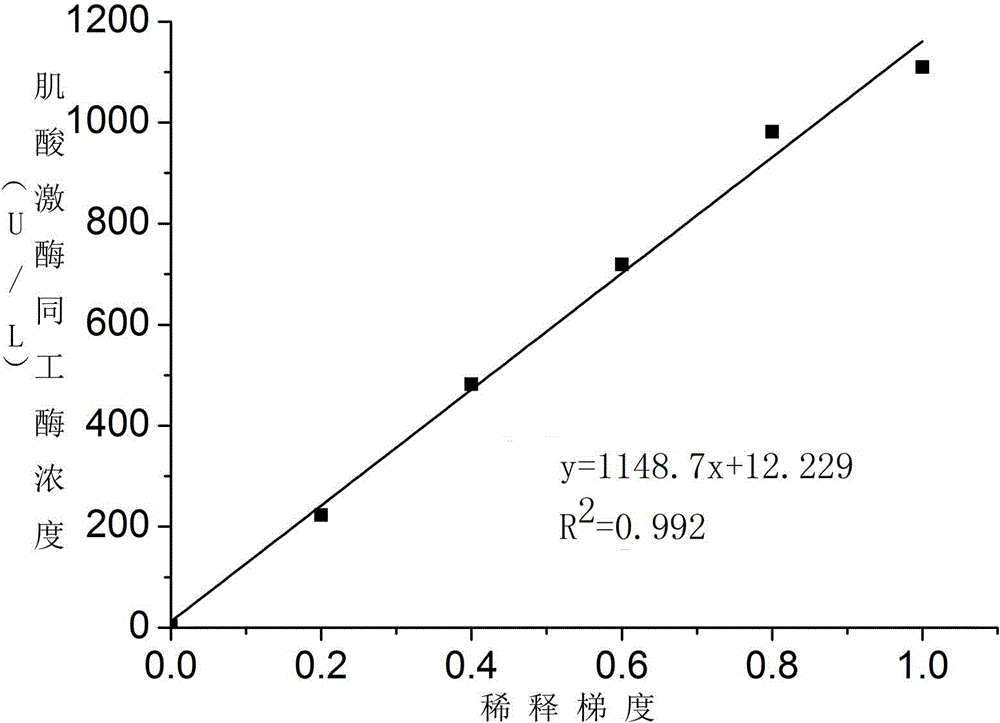
The invention provides a test paper used for detecting acute myocardial infarction, and a preparation method and an application method thereof. The test paper comprises a base plate and sequentially closely connected components adhered to the base plate, wherein the components are a sample absorption pad, a bonding pad, a chromatography membrane, and a water absorption pad. The bonding pad is coated with a glucan-antibody-fluorescein mixed marker. The chromatography membrane is provided with a detection band and a control band. A monoclonal antibody is fixed on the detection band. A rabbit anti-mouse IgG antibody which can be speficically bound with the glucan-antibody-fluorescein mixed marker is fixed on the control band. The sensitivity range of the reagent provided by the invention reaches 0.1ng / L. With the test paper, quantitative detections upon trace myoglobin, troponin I and creatine kinase isoenzyme in myocardial infarction patient serum can be realized. A result can be obtained in 3-5 minutes after sample spotting. The operation is simple, and professional operation is not needed.
Owner:武汉渊元医学科技有限公司
Creatine kinase isoenzyme detection reagent
ActiveCN104374925AHigh activityEfficient removalBiological testingSodium acetateAntiendomysial antibodies
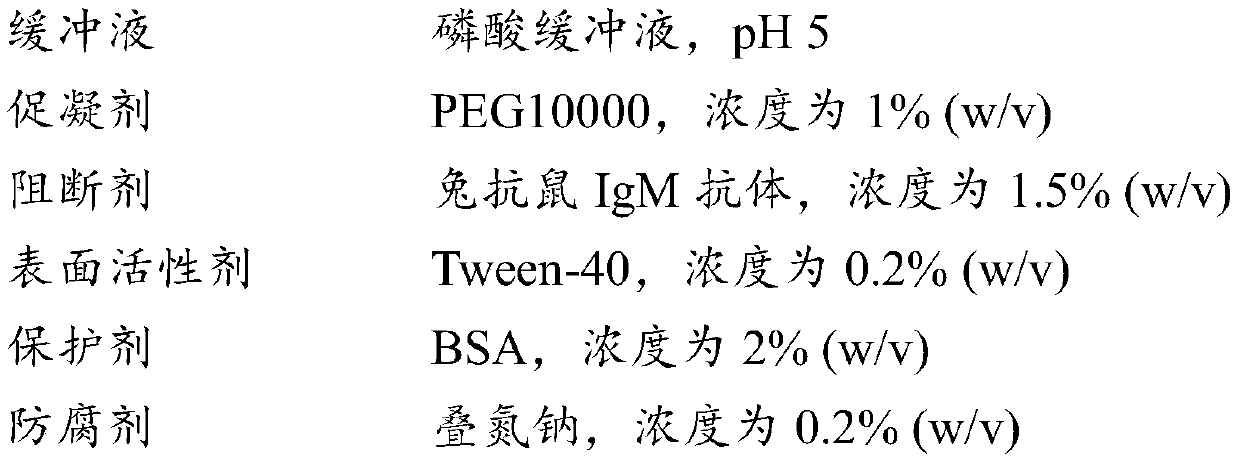
The invention discloses a creatine kinase isoenzyme detection reagent. The reagent consists of a reagent R1 and a reagent R2 according to the volume ratio of 4 to 1, wherein the reagent R1 consists of an imidazole buffer liquid, glucose, nano particles, N-acetylcysteine, sodium ethylene diamine tetracetate, adenosine diphosphate, cozymase I, ribonucleotide, pyruvate decarboxylase, glucose 6-phosphate dehydrogenase, hexokinase, a goat anti-human CK-M polyclonal antibody and trehalose; the reagent R2 consists of an imidazole buffer liquid, phosphocreatine, sodium dodecyl benzene sulfonate and a preservative. The reagent disclosed by the invention is a creatine kinase isoenzyme detection reagent which is stable, accurate, and high in anti-interference, and has very high clinical application value.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Screening kit and method for progressive muscular dystrophy of newborns
PendingCN111965352AAvoid hydrolysisReduce distractionsDisease diagnosisReference solutionsDiseaseHemolysis
The invention particularly discloses a double-antibody sandwich immunoassay in-vitro diagnostic reagent suitable for screening progressive muscular dystrophy of newborns. The double-antibody sandwichimmunoassay in-vitro diagnostic reagent is used for specifically detecting creatine kinase isoenzyme CK-MM. According to the invention, a filter paper dry blood slide calibrator and a filter paper dryblood slide sample are adopted and are not influenced by AK released by red blood cells during hemolysis, so that the universality of screening the progressive muscular dystrophy of newborns is realized. The invention discloses a filter paper dry blood slide calibrator preparation method, which solves the instability of the calibrator and prevent the hydrolysis of creatine kinase isoenzyme and / orsubtype by carboxyl peptidase in blood plasma. The invention discloses a special filter paper dry blood slide sample eluent, which solves the stability problem of a to-be-detected object during sample detection and reduces the interference of external factors. After muscular dystrophy is diagnosed in the early stage and proper treatment measures are taken, the progress of the disease can be greatly delayed, the life quality of a patient is improved, and huge cost caused by blind medical treatment of the patient can also be avoided.
Owner:GUANGZHOU FENGHUA BIOENG
Anti-human-CKMB (Hybrid Creatine Kinase Isoenzymes) antibody and application thereof
ActiveCN111349168AHigh activityHigh affinityDisease diagnosisFermentationAntiendomysial antibodiesAntigen binding
The invention relates to a novel separated binding protein comprising a CKMB (Hybrid Creatine Kinase Isoenzymes) antigen binding structural domain, and researches the aspects of preparation, application and the like of the binding protein. The binding protein has high activity, has a very high affinity with a human CKMB protein, and can be widely applied to the field of detection of the CKMB protein.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
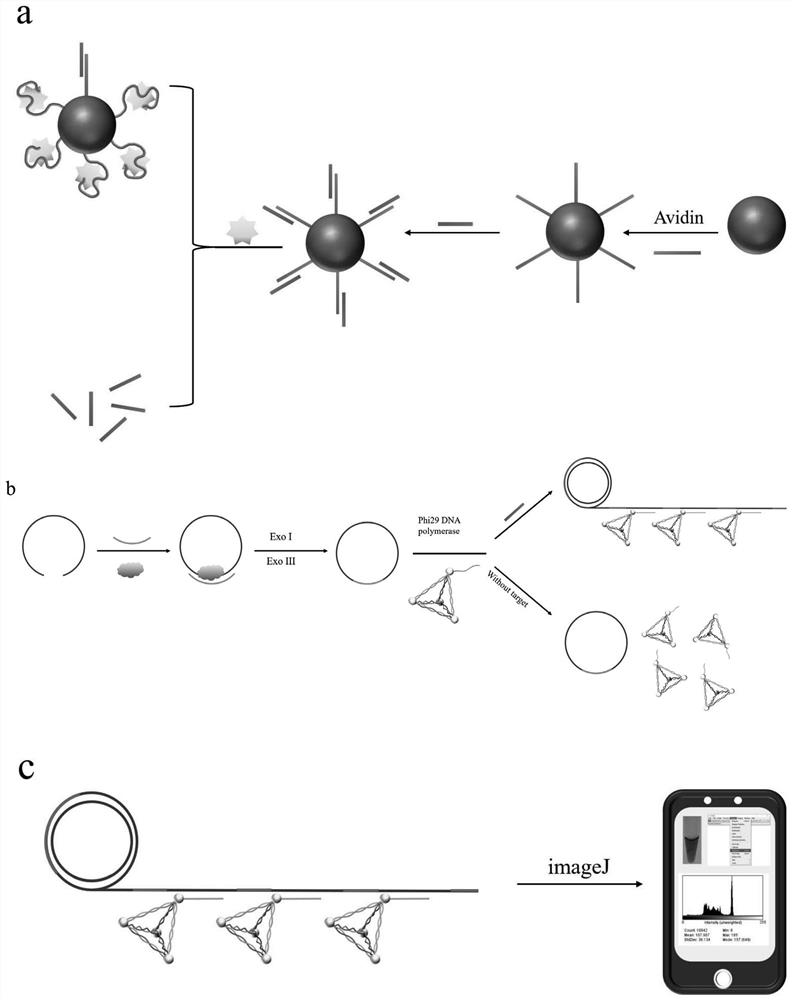
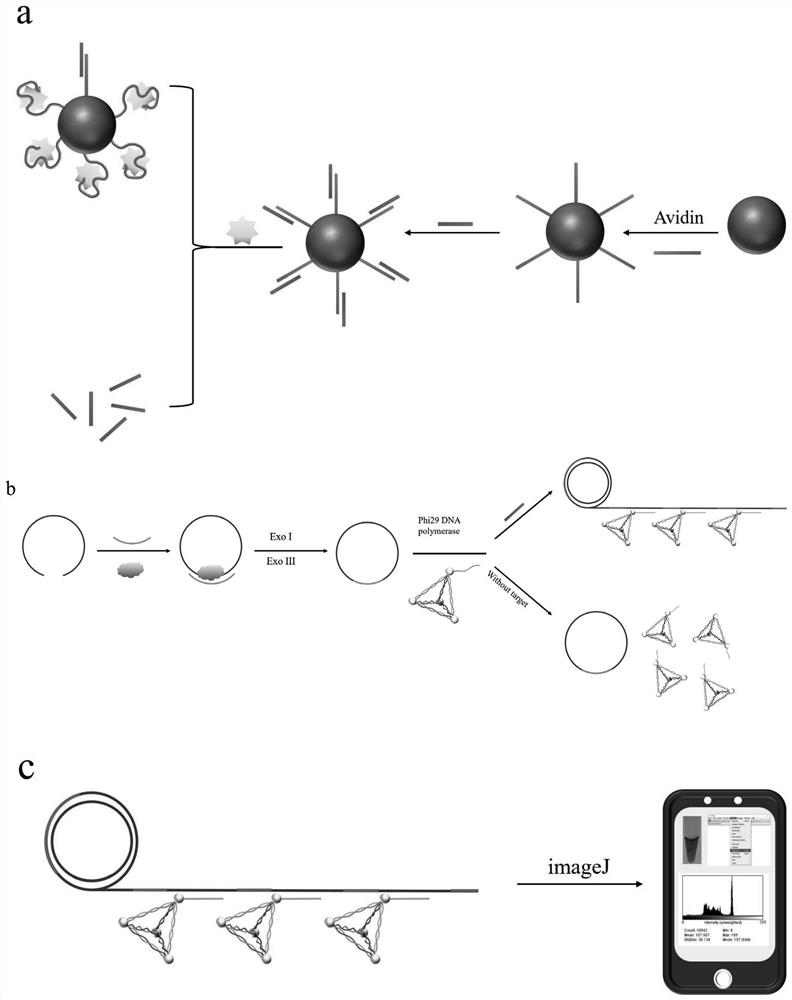
Rolling circle amplification-gold tetrahedron colorimetric detection method and kit for detecting creatine kinase isoenzyme
ActiveCN113265447ASensitive and accurate detectionImprove stabilityMicrobiological testing/measurementBiological material analysisProtein detectionMagnetic bead
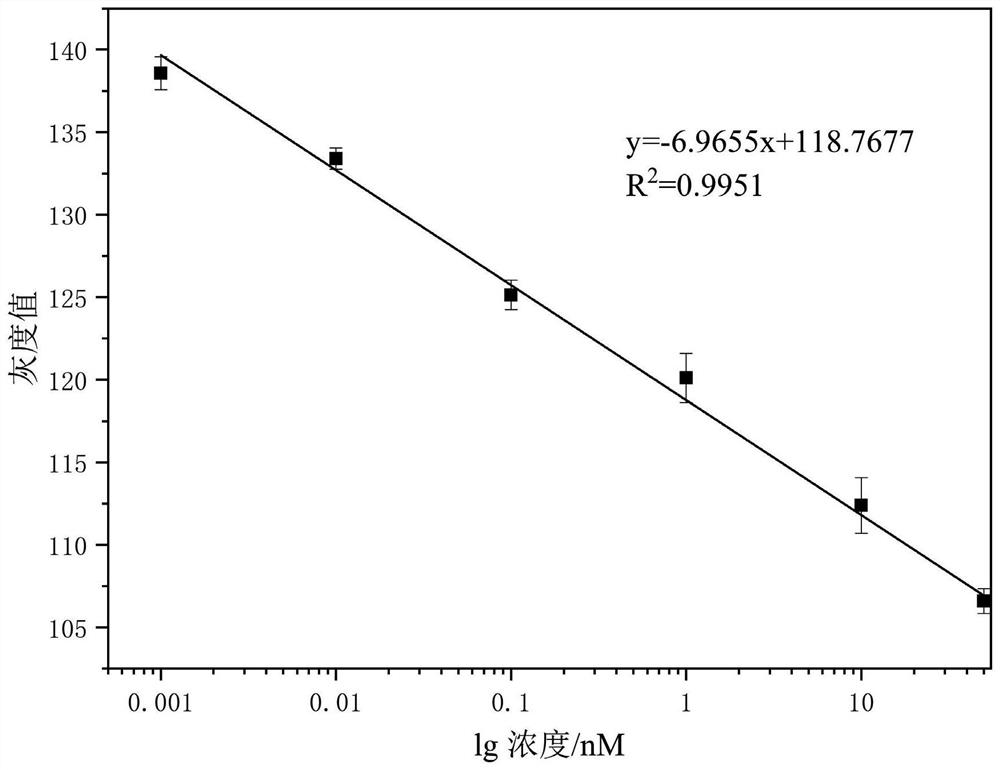
The invention belongs to the field of protein detection, and relates to a rolling circle amplification-gold tetrahedron colorimetric detection method and kit for detecting creatine kinase isoenzyme. The detection method comprises the following steps: S1, preparing a magnetic bead-aptamer-complementary chain compound; S2, preparing a DNA-AuNPs compound; S3, preparing an AuNPs tetrahedron; S4, preparing a rolling circle amplification template; S5, carrying out competitive reaction on creatine kinase isoenzyme and a complementary chain; S6, performing rolling circle amplification; S7, carrying out aggregation chromogenic reaction on the gold tetrahedron; and S8, conducting reading. According to the invention, an isothermal amplification technology is utilized, a complicated temperature change process is not needed, stability is relatively good, and sensitivity is relatively high. Compared with other detection methods, the colorimetric method disclosed by the invention does not need a large instrument for detection, can obtain a detection result through imaginJ software, is relatively low in cost, has a detection limit of 0.05 pM, and is suitable for on-site screening and rapid detection processes.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
Microfluidic chip colorimetric detection method and kit for detecting creatine kinase isoenzyme
PendingCN113311161AQuick Visual InspectionReduce manufacturing costMaterial analysis by observing effect on chemical indicatorDisease diagnosisProtein detectionNanoparticle
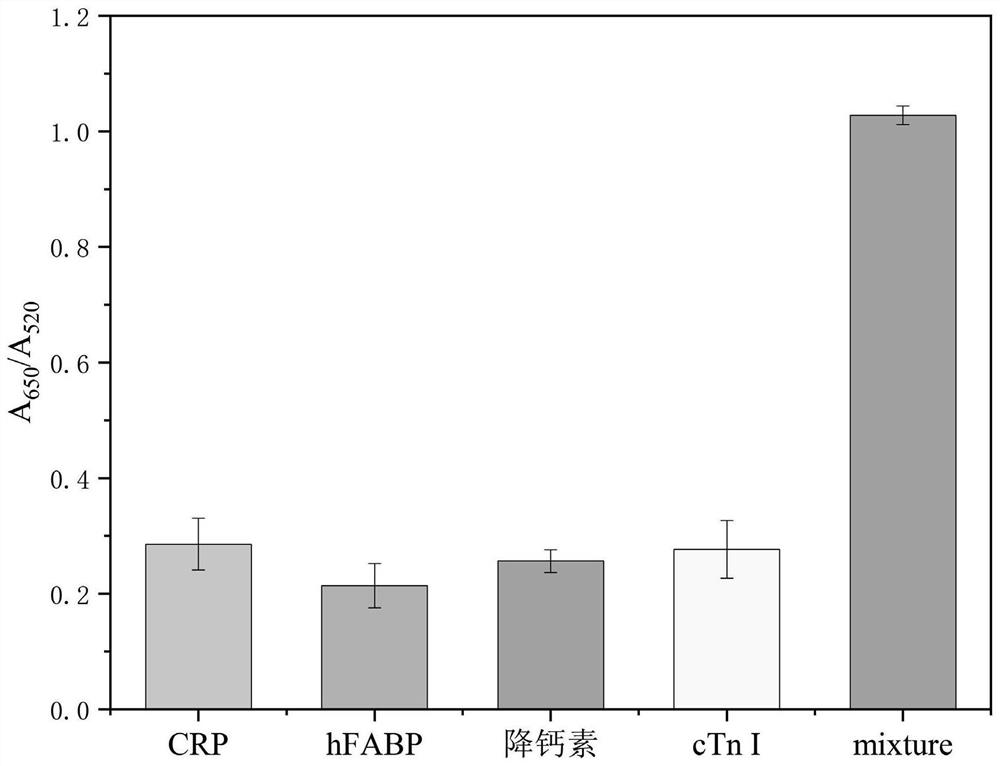
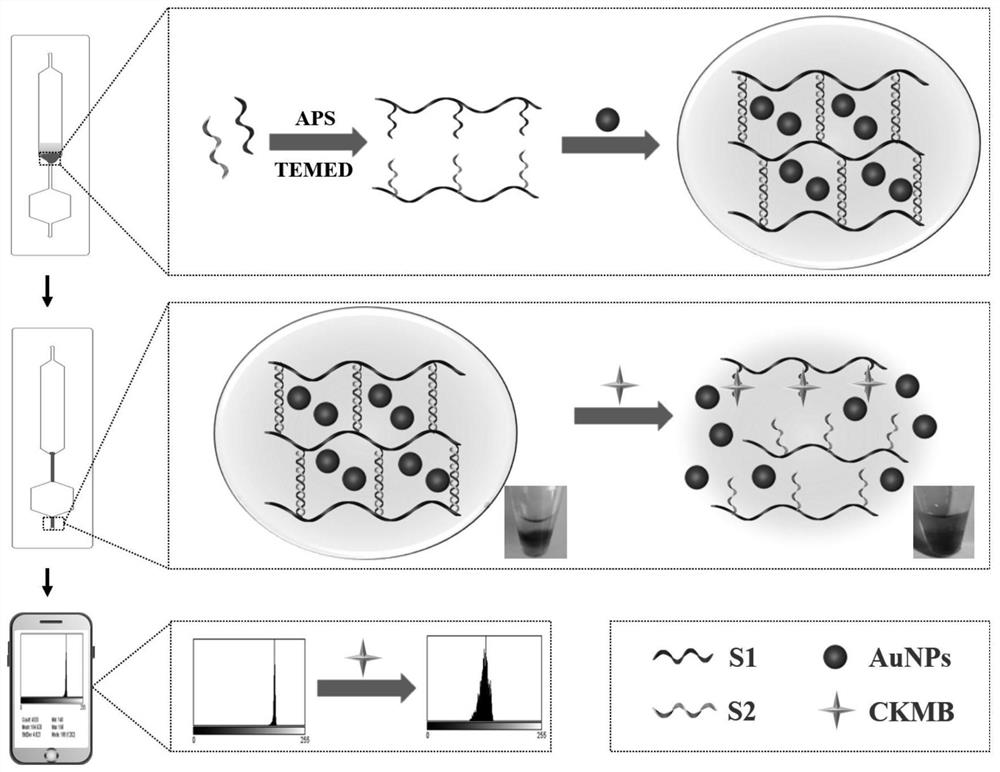
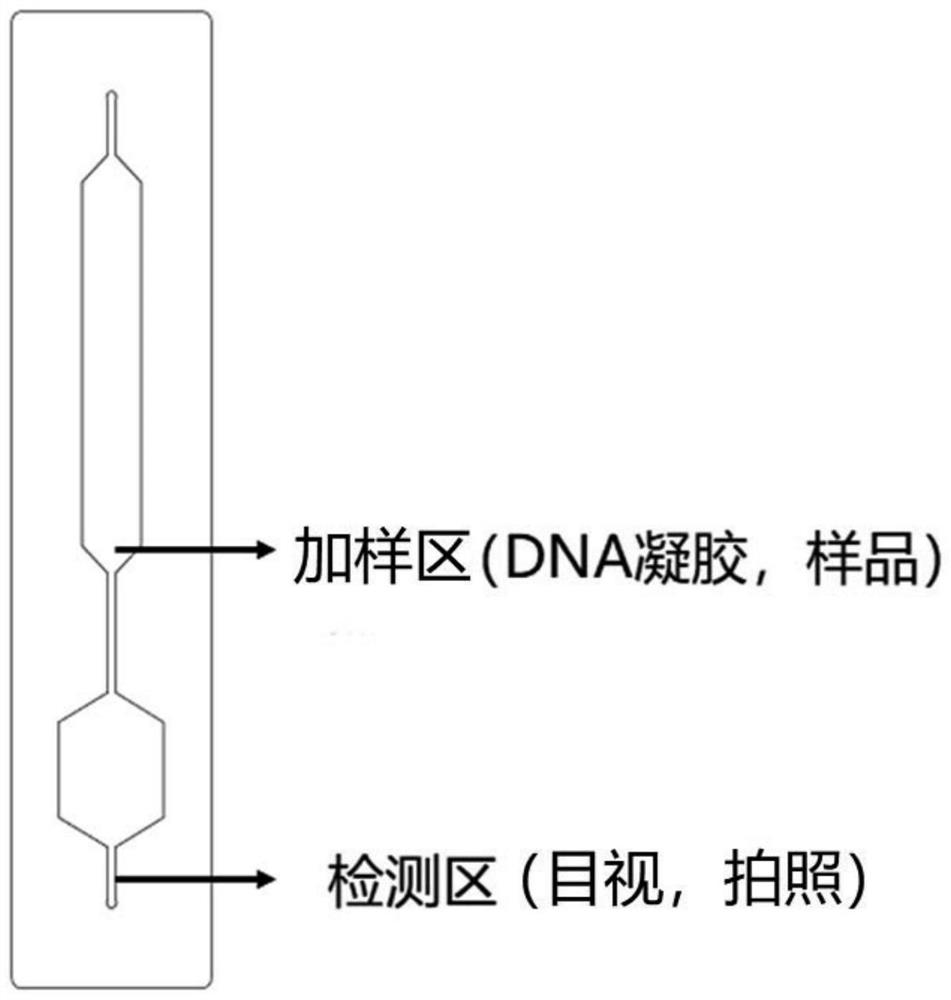
The invention belongs to the field of protein detection, and relates to a microfluidic chip colorimetric detection method and kit for detecting creatine kinase isoenzyme. The method comprises the following steps: S1, preparing a linear polyacrylamide-DNA polymer; S2, synthesizing and modifying gold nanoparticles; S3, designing and assembling a micro-fluidic chip; S4, synthesizing DNA hydrogel; and S5, detecting. According to the invention, the DNA hydrogel is used for detecting the target object, the stability is good, the reaction specificity is high, and visual detection can be achieved; and the micro-fluidic chip is used for detection, and the purpose of portable quantitative detection is achieved.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
Creatine kinase isoenzyme CK-MB detection kit and detection method thereof
PendingCN113502318AImprove accuracyImprove stabilityMicrobiological testing/measurementAntiendomysial antibodiesAdenosine diphosphate
The invention relates to the technical field of in-vitro diagnostic reagents, in particular to a creatine kinase isoenzyme CK-MB detection kit and a detection method thereof. The creatine kinase isoenzyme CK-MB detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises chromium nicotinate, an imidazole buffer solution, diadenosine pentaphosphate (AP5A), adenosine monophosphate (AMP), glucose-6-phosphate dehydrogenase, magnesium acetate, glucose, nicotinamide adenine dinucleotide phosphate (NADP <+>) and an anti-CK-MB antibody; the R2 reagent comprises an imidazole buffer solution, adenosine diphosphate (ADP), phosphocreatine, sodium azide and glucokinase. Meanwhile, the invention further provides a method for detecting the content or concentration of the creatine kinase isoenzyme CK-MB, the method adopts the creatine kinase isoenzyme CK-MB detection kit to detect the content or concentration of the creatine kinase isoenzyme CK-MB, and the detection accuracy can be effectively improved.
Owner:WHITMAN BIOTECH NANJING
High-sensitivity creatine kinase isoenzyme detection kit and preparation method thereof
PendingCN112540177AHigh sensitivityStrong specificityBiological material analysisAntiendomysial antibodiesMicrosphere
The invention provides a high-sensitivity creatine kinase isoenzyme detection kit and a preparation method thereof. The high-sensitivity creatine kinase isoenzyme detection kit comprises a reagent R1and a reagent R2; the preparation method comprises the steps: reacting an anti-mouse IgG-Fc antibody with amino groups on the surfaces of latex microspheres to form a shift alkali, directionally marking the latex microspheres with the anti-mouse IgG-Fc antibody, connecting the anti-mouse IgG-Fc antibody with at least two mouse anti-human CK-MB monoclonal antibodies, and coupling the latex microspheres with the mouse anti-human CK-MB monoclonal antibodies to prepare a latex microsphere reagent; and adding the latex microsphere reagent into a closed buffer solution, carrying out resuspension ultrasonic treatment, closing, adding a latex preservation solution, carrying out resuspension ultrasonic treatment to prepare latex reagents, and mixing at least two latex reagents to prepare a reagentR2. Compared with the prior art, the kit is high in sensitivity, wide in detection range, good in repeatability, high in specificity and good in stability; compared with other coupling methods, the method provided by the invention has the advantages that the CK-MB monoclonal antibody F (ab) end active sites are prevented from being coupled to the microspheres, meanwhile, the CK-MB monoclonal antibody coupling amount is increased, and the reagent detection sensitivity is obviously improved.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Anti-creatine kinase isoenzyme antibody as well as preparation method, application and amino acid sequence thereof
ActiveCN111606997AStrong specificityHigh affinityPeptide preparation methodsDisease diagnosisHeavy chainImmunologic Technique
The invention relates to the technical field of immunology, and particularly discloses an anti-creatine kinase isoenzyme antibody and a preparation method, application and amino acid sequence thereof.The anti-creatine kinase isoenzyme antibody comprises an amino acid sequence of a variable region of a heavy chain, wherein the amino acid sequence comprises a sequence shown in SEQ ID NO: 1; and anamino acid sequence of a variable region of a light chain, wherein the amino acid sequence comprises a sequence shown in SEQ ID NO: 2. The sequence shown as SEQ ID NO: 1 and the sequence shown as SEQID NO: 2 have at least one substitution. The antibody has the characteristics of high specificity and strong affinity.
Owner:杭州博岳生物技术有限公司
Creatine kinase isoenzyme MB detection kit and preparation method thereof
The invention provides a creatine kinase isoenzyme MB detection kit and a preparation method thereof. Specifically, the kit comprises a creatine kinase isoenzyme MB detection reagent card, and the reagent card comprises a sample pre-adding part and a test strip; wherein the pre-sampling part comprises a sampling container and a creatine kinase isozyme MB monoclonal antibody-fluorescent microsphereconjugate which is pre-added into the sampling container; the test strip comprises a test strip bottom plate, and a sample loading part, a detection part and a liquid absorption part which are arranged on the test strip bottom plate, wherein the detection part comprises a chromatography matrix, a detection band and a quality control band, wherein the detection band and the quality control band are respectively arranged, the detection band is coated with a creatine kinase isozyme CK-MB polyclonal antibody, and the quality control band is coated with an IgG polyclonal antibody. The kit is simple to operate, low in cost, good in repeatability and high in accuracy.
Owner:上海艾瑞德生物科技有限公司
Application of a human amniotic mesenchymal stem cell
ActiveCN105769910BImproved flow dynamicsProtect against pathological changesUnknown materialsSkeletal/connective tissue cellsCardiac functioningAcyl CoA dehydrogenase
The invention discloses application of human amniotic mesenchymal stem cells in preparation of a preparation for treating a myocardial ischemia reperfusion injury generated after cardiopulmonary bypass.In the myocardial ischemia reperfusion process after the cardiopulmonary bypass, the human amniotic mesenchymal stem cells can effectively prevent myocardial cells from being injured in myocardial ischemia reperfusion, obviously improve the cardiac functions, lower the levels of myocardial injury specific marker protein lactic dehydrogenase, creatine kinase isoenzyme and troponin I, lower the levels of a plasma inflammatory factor interleukin-8 and a tumor mecrosis factor-alpha, increase the content of the plasma inflammatory factor interleukin-10, obviously improve pathological changes of the myocardial tissue, reduce myocardial cell apoptosis, lower the expression level of cell apoptosis promoting protein, increase the expression quantity of anti-apoptoasis protein and can protect the functions of myocardial cell mitochondria.By preparing the human amniotic mesenchymal stem cells into an injection for treatment on the myocardial ischemia reperfusion injury generated after the cardiopulmonary bypass, the cardiac functions can be effectively protected, and postoperative complications of the cardiopulmonary bypass are relieved.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Preparation method of in vitro diagnosis test paper strip based on CdSe quantum dots as marker
The invention relates to a preparation method of an in vitro diagnosis test paper strip based on CdSe quantum dots as a marker, and belongs to the field of biotechnology, wherein the preparation method comprises: preparation of CdSe quantum dots, coupling of quantum dots and primary antibody, and preparation of myocardial in vitro diagnosis test paper strip. According to the present invention, with the preparation method of the in vitro diagnosis test paper strip, the contents of troponin I, myoglobin and creatine kinase isoenzyme in whole blood, serum and plasma can be quickly and quantitatively detected so as to provide the basis for the early stage diagnosis of myocardial diseases; and the preparation method has advantages of simple operation, high efficiency, low cost, easy automationand the like.
Owner:北京盛坤康如医疗器械有限责任公司
Fluorescence sensing method and kit for simultaneously detecting cortisol, serum testosterone and creatine kinase isoenzyme
ActiveCN113804898ASensitive and accurate detectionImprove stabilityMicrobiological testing/measurementBiological material analysisCortisol serumMagnetic bead
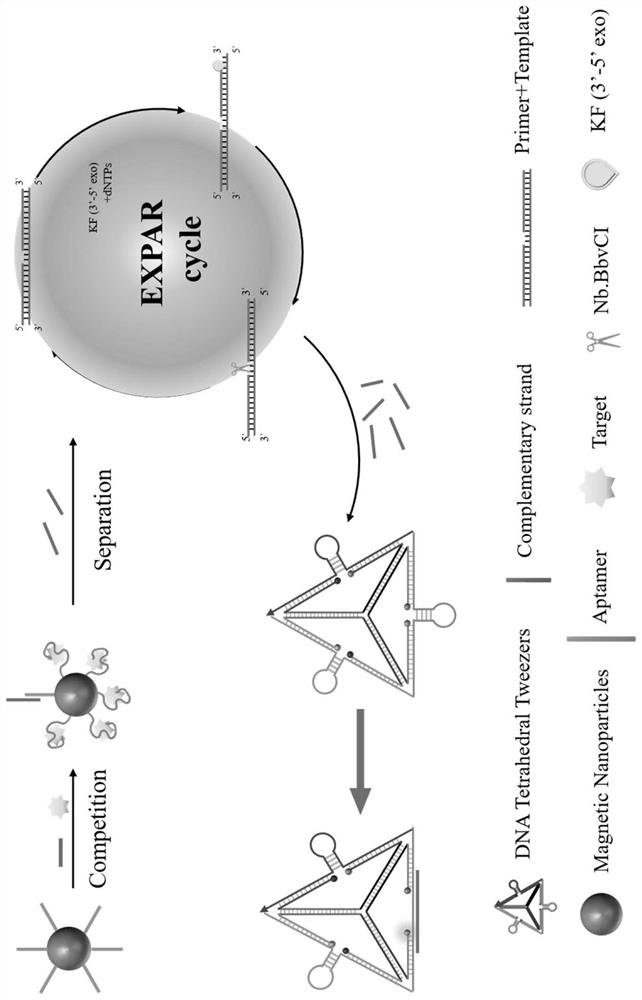
The invention discloses a fluorescence sensing method and kit for simultaneously detecting cortisol, serum testosterone and creatine kinase isoenzyme, and belongs to the field of biomarker detection. The fluorescence sensing method comprises the following steps: (1), preparing a magnetic bead-aptamer-complementary chain compound; (2), preparing DNA tetrahedral tweezers; (3), performing competition between the target object and the complementary chain; (4), carrying out EXPAR amplification reaction; (5), carrying out complementary chain-DNA tweezers reaction; and (6), performing reading: measuring the fluorescence value of the reaction system. The EXPAR isothermal amplification technology is utilized, a complex temperature change process is not needed, stability is good, sensitivity is high, compared with other detection methods, the content of three target substances including testosterone, cortisol and creatine kinase isoenzyme in serum can be detected at the same time, the content of the target substances is detected according to the fluorescence recovery degree, cost is low. And the detection limits can respectively reach 41pM, 68pM and 8pM.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
Kit for specifically detecting creatine kinase isoenzyme
ActiveCN113189337AHigh sensitivityHigh detection specificityDisease diagnosisBiological testingAntiendomysial antibodiesActive agent
The invention discloses a kit for specifically detecting creatine kinase isoenzyme and belongs to the technical field of creatine kinase detection. The kit comprises a reagent R0, a reagent RM and a reagent R1, wherein the reagent R0 comprises a buffer solution, sodium chloride, a protective agent, a preservative and a creatine kinase isoenzyme B subunit antibody; the reagent RM comprises a buffer solution, magnetic microspheres coated with a creatine kinase MM isoenzyme coating antibody, a protective agent, a surfactant, a stabilizer and a preservative; the reagent R1 comprises a buffer solution, a creatine kinase MM isoenzyme labeled antibody coated with alkaline phosphatase, a protective agent, a surfactant, a stabilizer and a preservative. The kit is high in sensitivity and good in specificity, has a relatively large linear range and HOOK resistance, can detect CKMM in a dry filter blood sheet, serum or urine sample, and is relatively wide in application range.
Owner:BEIJING DIAGREAT BIOTECH CO LTD
Kit for simultaneously detecting multiple biomarkers based on nucleic acid rolling circle amplification reaction
PendingCN112557666AFull "sandwich" responseFully take place "sandwich" reactionMicrobiological testing/measurementDisease diagnosisMagnetic beadRNA - Ribonucleic acid
The invention provides a kit for simultaneously detecting multiple biomarkers based on nucleic acid rolling circle amplification reaction, and relates to the technical field of marker detection. The kit provided by the invention comprises four independent components: a specific antibody solution for labeling single-stranded deoxyribonucleic acid; a magnetic bead suspension coated with a specific antibody of the biomarker; a reaction solution containing cyclic deoxyribonucleic acid; and reaction solutions containing different probes. When the kit disclosed by the invention is used for detectingvarious markers, the detection result is similar to the detection result of Japonica chemiluminiscence, even lower concentration (ag / ml) can be detected within enough time, and the sensitivity is obviously superior to that of a chemiluminiscence detection method. For example, when cardiovascular disease biomarkers such as cardiac troponin I (cTnI), myoglobin (Myo) and creatine kinase isozyme (CKMB) are detected, detection of three indexes is completed in one reaction cup at the same time, and results are not interfered with one another.
Owner:湖南博奥瑞康生物科技有限公司
Latex enhanced immunoturbidimetry kit for detecting creatine kinase isoenzyme CK-MB
ActiveCN111562372AImprove anti-interference abilityHigh sensitivityDisease diagnosisMicrosphereActive agent
The invention provides a latex enhanced immunoturbidimetry kit for detecting creatine kinase isoenzyme CK-MB. The latex enhanced immunoturbidimetry kit comprises a reagent R1 and a reagent R2, whereinthe reagent R1 comprises a buffer solution, a coagulant, a blocking agent, a surfactant, a protective agent and a preservative, and the reagent R2 comprises a buffer solution, CK-MB antibody coated nano microspheres, a protective agent and a preservative. A rabbit anti-mouse IgM antibody or a SeaBlock fish plasma blocking agent is adopted as the blocking agent in the reagent 1, and glycine, ethanolamine and calf serum are adopted as a sealing agent in the CK-MB antibody coated nano microspheres of the reagent 2, so that the detection interference can be effectively reduced. The kit provided by the invention has the advantages of strong anti-interference performance, high sensitivity, wide detection range, good repeatability, high specificity, good stability and the like, can realize automatic detection on a biochemical analyzer, replace a chemiluminescence product and reduce the detection cost, and meets clinical use.
Owner:SHENZHEN AMTECH BIOENGINEERING LTD INC
CK-MB type creatine kinase isozyme and preparation method and application thereof
InactiveCN110904068AHigh yieldImprove biological activityMicrobiological testing/measurementTransferasesAntigenAntiendomysial antibodies
The invention belongs to the technical field of enzyme engineering, and particularly relates to a CK-MB type creatine kinase isozyme and a preparation method and application thereof. The CK-MB type creatine kinase isozyme obtained by the preparation method provided by the invention is relatively high in yield, and the yield of M subunit protein and B subunit protein after equal-proportion renaturation is 35mg / L. The CK-MB type creatine kinase isoenzyme obtained by the preparation method disclosed by the invention performs quantitative detection by using a Roche creatine kinase isoenzyme detection kit (an electrochemical luminescence method), and the activity rate of the CK-MB type creatine kinase isoenzyme is greater than 41%. The preparation method of the CK-MB type creatine kinase isoenzyme provided by the invention is simple to operate, low in cost and high in yield. The prepared CK-MB type creatine kinase isoenzyme is high in biological activity and good in stability, can be used as a quality control product or a calibration product in in-vitro diagnosis, and can also be used as immunogen or screening antigen for creatine kinase isoenzyme antibody development.
Owner:杭州博茵生物技术有限公司
Creatine kinase isoenzyme (CK-MB) detection kit capable of resisting heparin interference
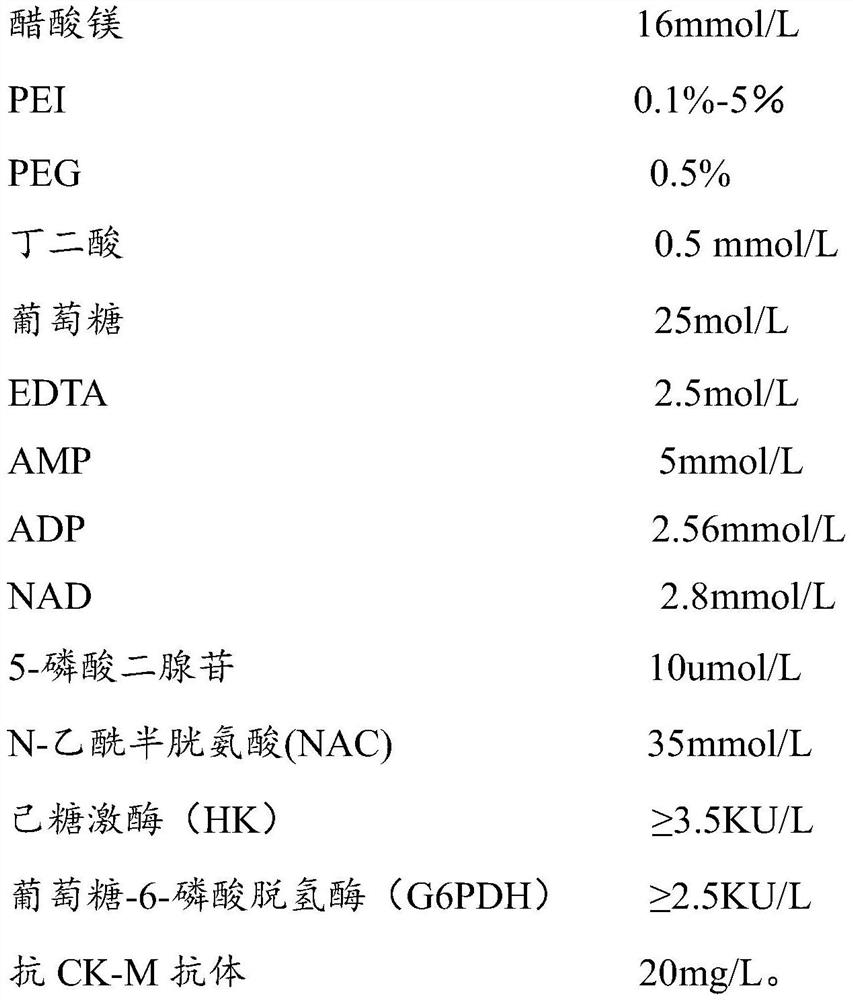
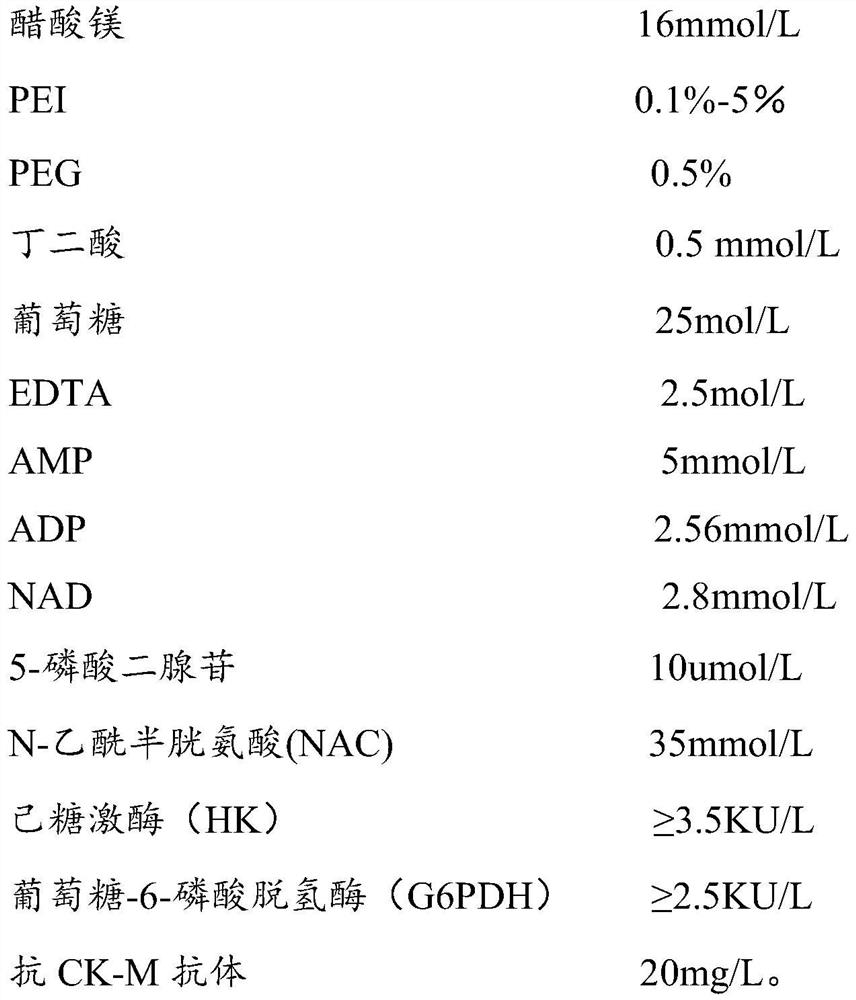
PendingCN112379091AEliminate distractionsImprove stabilityBiological material analysisBiochemical diagnosisCreatine kinase isoenzymes
The invention belongs to the technical field of creatine kinase isoenzyme (CK-MB) biochemical diagnosis, and discloses a creatine kinase isoenzyme (CK-MB) detection kit and a creatine kinase isoenzyme(CK-MB) detection method for resisting heparin interference. The detection kit comprises an R1 reagent and an R2 reagent, the R1 reagent comprises PEI or hydrophilic macromolecule modified PEI, and interference caused by heparin contained in a sample can be eliminated. Through test detection, compared with a serum tube, a heparin anticoagulant tube determination result is not too high and is consistent with a serum tube result by adding amine modified by a modification group into the reagent, so that the accuracy of the creatine kinase isoenzyme (CK-MB) detection kit is improved, and the stability of the kit is improved.
Owner:SHANGHAI KEHUA BIO ENG
Creatine kinase isoenzyme detection kit
PendingCN114487420AImprove anti-interference abilityHigh sensitivityBiological material analysisAntiendomysial antibodiesActive agent
The invention discloses a creatine kinase isoenzyme detection kit, and belongs to the technical field of biological detection. The creatine kinase isoenzyme detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises the following components: a buffer solution, a coagulant, a chelating agent, a blocking agent, a surfactant, a stabilizer and a preservative; and the reagent R2 comprises the following components: a buffer solution, latex particles coated with creatine kinase isoenzyme antibodies, a sealing agent, a stabilizer and a preservative. The creatine kinase isoenzyme detection kit disclosed by the invention overcomes the defects of the existing creatine kinase isoenzyme detection kit, and has the advantages of strong anti-interference performance, high sensitivity, high specificity, high accuracy, wide linear range, good stability and the like; and a safe, rapid, accurate and pollution-free detection means is provided for the detection of the creatine kinase isoenzyme.
Owner:广西康柏莱科技有限公司
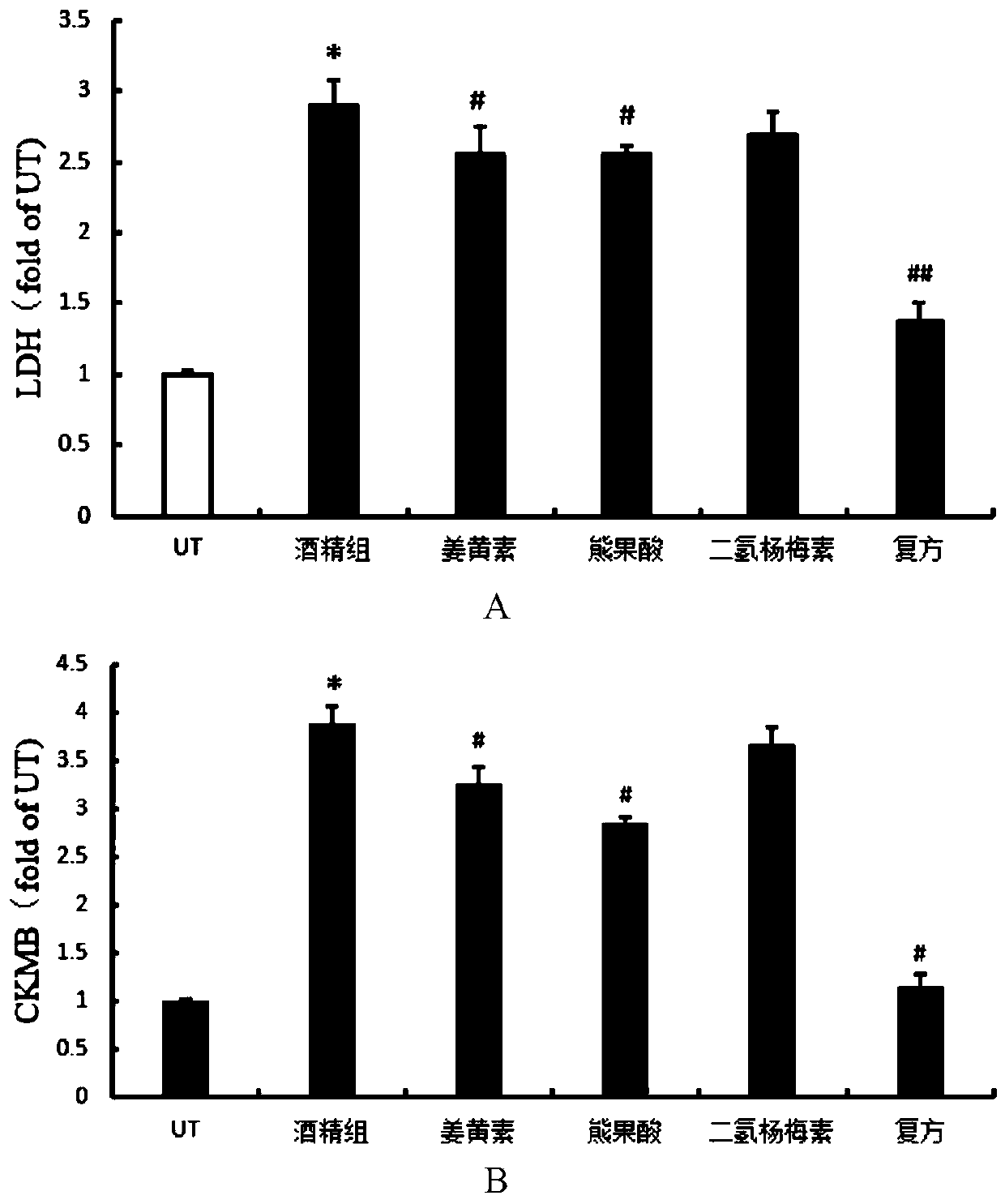
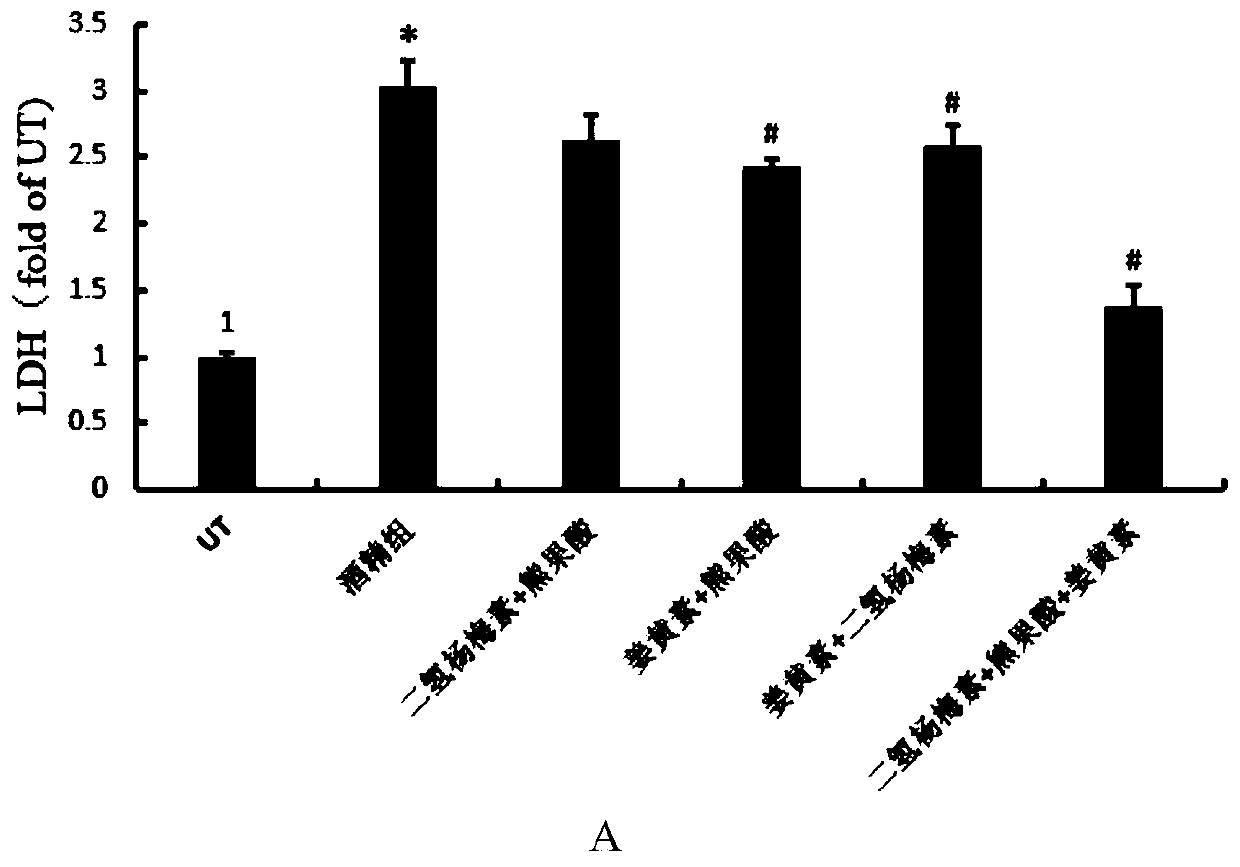
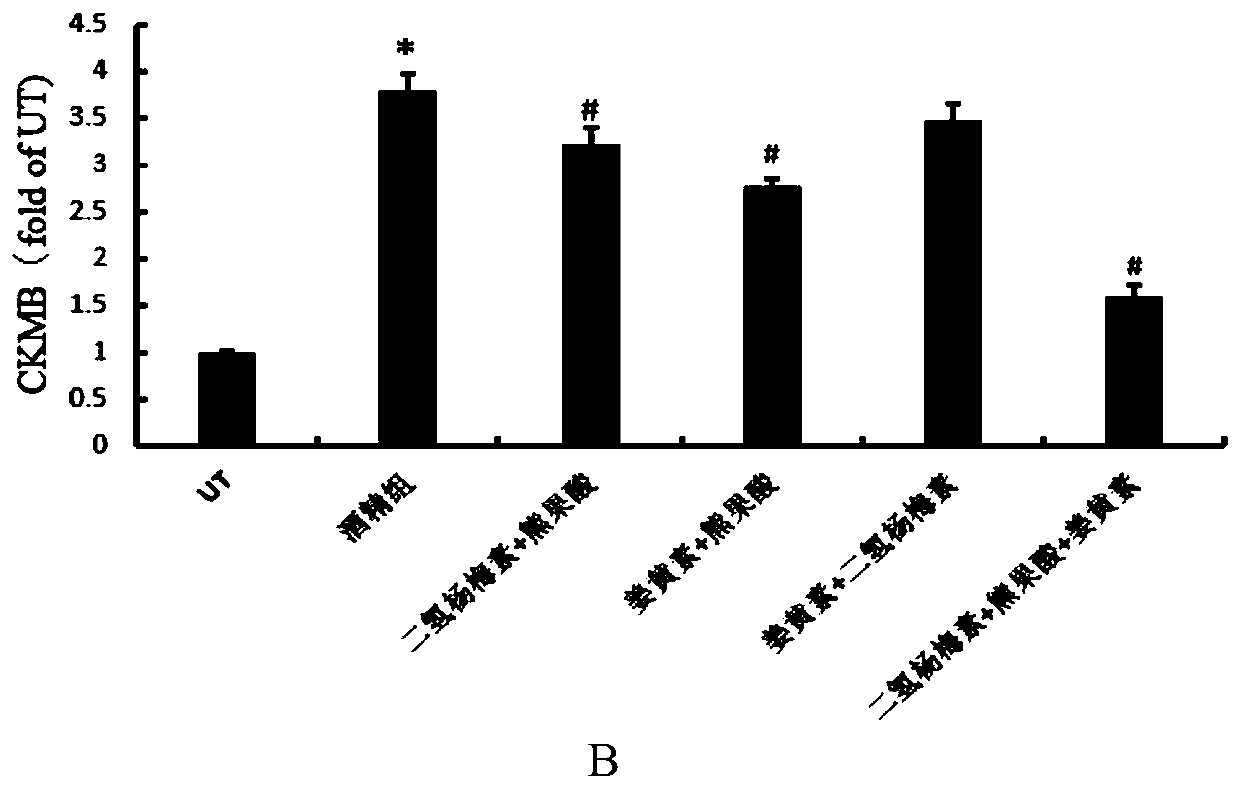
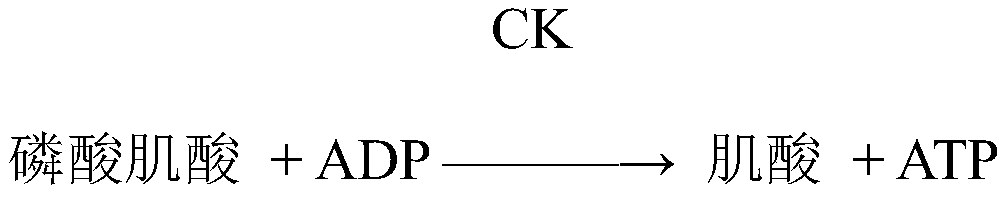
A compound composition capable of reducing the content of cardiac creatine kinase isozyme and its application
ActiveCN108653269BPlasma CK-MB content decreasedRestore blood ejection abilityKetone active ingredientsCardiovascular disorderBlood plasmaBiology
The invention discloses a compound composition with the function of decreasing the cardiac CK-MB content and application thereof. According to the compound composition, the plasma CK-MB content in analcoholic cardiomyopathy model is decreased by 46.86%, the cardiac ejection capability in alcoholic cardiomyopathy is restored, the myocardial hypertrophy is alleviated, and the myocardial damage caused by the alcoholic cardiomyopathy model is remarkably reduced; at present, clinical manifestation of the alcoholic cardiomyopathy is often accompanied by cardiac dilatation and congestive heart failure, mainly left ventricular failure; and the compound composition can effectively restore and increase the left ventricle posterior wall thickness and enable a patient to recover from myocardial damage, so that the patient can be effectively recovered from the cardiac damage situation caused by an alcoholic diet model.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Phosphocreatine kinase isoenzyme detection kit
PendingCN111157724AHigh sensitivityGood repeatabilityBiological material analysisAntiendomysial antibodiesEthylic acid
The invention discloses a phosphocreatine kinase isoenzyme detection kit, and relates to the technical field of biomedicine. The kit includes a first reagent, wherein the first reagent comprises the following components: imidazole with concentration of 124 to 126mmol / L; magnesium acetate with concentration of 2 to 3mmol / L; ethylenediamine tetraacetic acid with concentration of 12-13 mmol / L; mannitol with concentration of 34 to 34.3 mmol / L; adenosine monophosphate with concentration of 6 to 6.5 mmol / L; lithium diadenosine pentaphosphate with concentration of 0.01 to 0.015 mmol / L, reductive coenzyme II with concentration of 2 to 3mmol / L, Proclin150 with concentration of 0.8 to 1.2 g / L, hexokinase with concentration of 3.5 to 4ku / L, N-acetylcysteine with concentration of 24 to 26mmol / L, sodium sulfite with concentration of 2 to 3mmol / L, and anti-human CK-M antibody with concentration of 2 to 3 ml / L. The improved phosphocreatine kinase isoenzyme detection kit provided by the invention is higher in sensitivity, better in repeatability, higher in accuracy and better in stability.
Owner:SHANGHAI HIGH TRACK MEDICAL EQUIP CO LTD
Kit for determining activity of creatine kinase isoenzyme and determination method thereof
PendingCN113866410AHigh test sensitivityBiological material analysisAntiendomysial antibodiesTetrazole
The embodiment of the invention belongs to the field of medical examination and determination, and relates to a kit for determining activity of creatine kinase isoenzyme. The kit comprises a first reagent and a second reagent; the first reagent comprises a first buffer solution, adenosine diphosphate, disodium ethylene diamine tetraacetate, magnesium acetate, an activator, adenosine monophosphate, an anti-human CK-MM antibody, AP5A, glucose, NAD (P) + and a first preservative; and the second reagent comprises the following components: a second buffer solution, phosphocreatine, magnesium acetate, hexokinase, glucose-6-phosphate dehydrogenase, tetrazolium salt, diaphorase and a second preservative. The invention also relates to a determination method of creatine kinase isoenzyme activity. According to the technical scheme, the test sensitivity can be improved.
Owner:DAAN GENE CO LTD
Magnetic particle chemiluminescent microfluidic chip for detection of creatine kinase isozyme in whole blood
ActiveCN105435867BLow backgroundHigh sensitivityLaboratory glasswaresMaterial analysisWhole blood unitsLiquid storage tank
Owner:SHENZHEN HUAMAIXINGWEI MEDICAL TECH CO LTD
A rolling circle amplification-gold tetrahedron colorimetric detection method and kit for detecting creatine kinase isoenzymes
ActiveCN113265447BSensitive and accurate detectionImprove stabilityMicrobiological testing/measurementBiological material analysisProtein detectionMagnetic bead
The invention belongs to the field of protein detection, and relates to a rolling circle amplification-gold tetrahedron colorimetric detection method and a kit for detecting creatine kinase isoenzymes. The method comprises the following steps: S1. preparing magnetic bead-adapter-complementary chain complex; S2. preparing DNA-AuNPs complex; S3. preparing AuNPs tetrahedron; S4. preparing rolling circle amplification template; S5. creatine kinase Competitive reaction between enzyme and complementary strand; S6. Rolling circle amplification; S7. Gold tetrahedron aggregation color reaction; S8. Readout. The invention utilizes the isothermal amplification technology, does not need complicated temperature changing process, has good stability and high sensitivity. Compared with other detection methods, the colorimetric method of the present invention does not require large-scale instrument detection, and the detection results can be obtained by imaginJ software, the cost is low, and the detection limit can reach 0.05pM, which is suitable for on-site screening and rapid detection process.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
Sandwich immunoassay kit
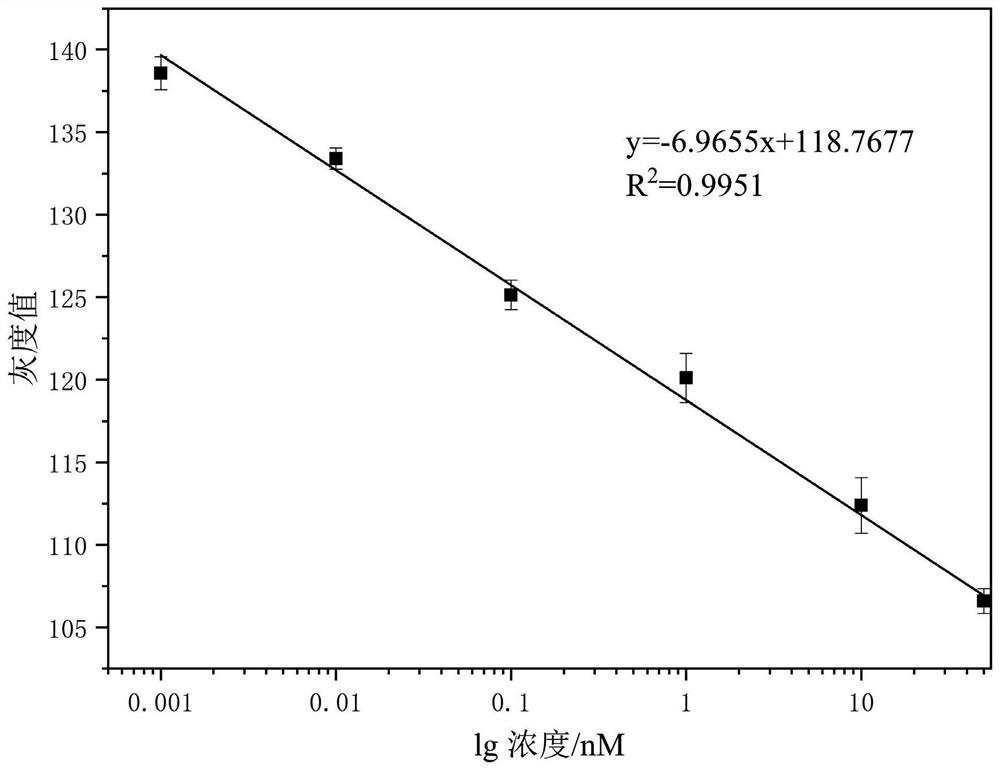
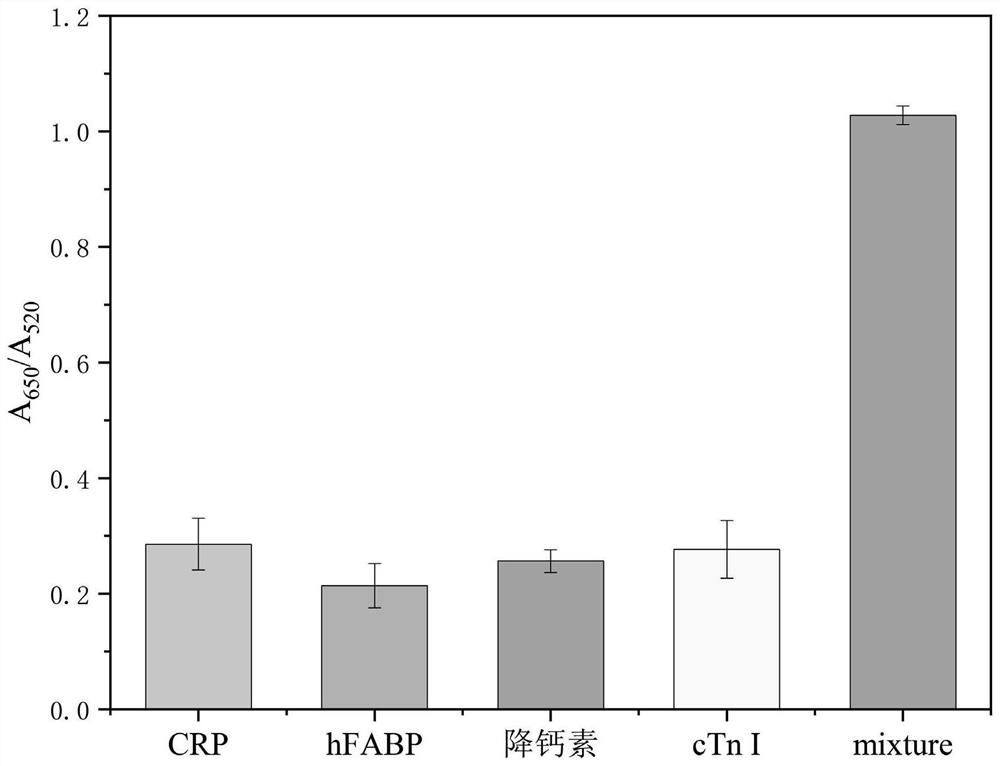
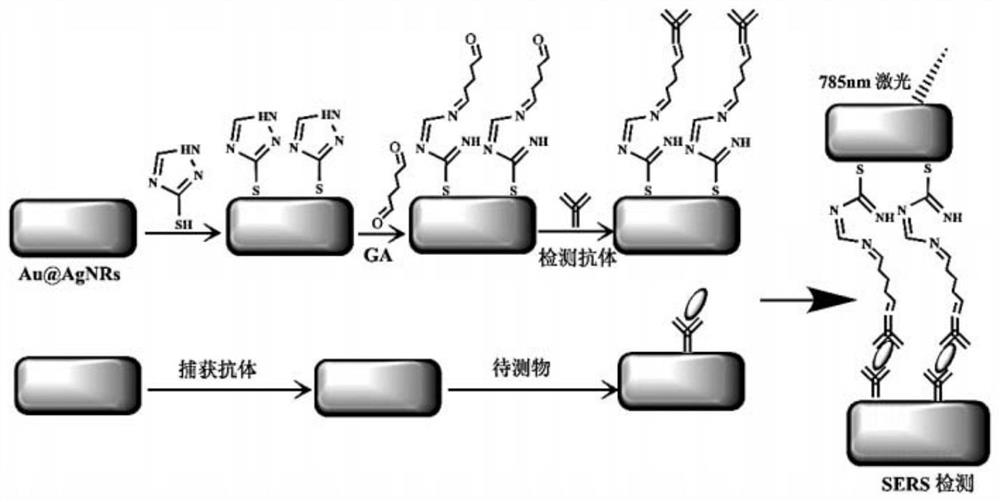
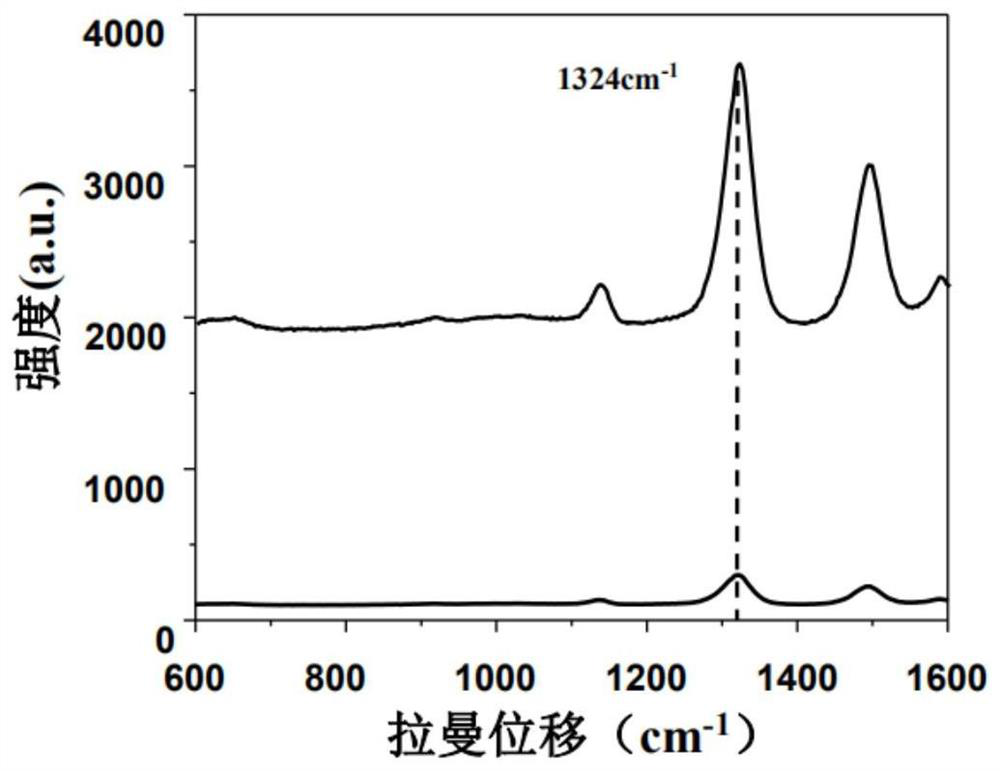
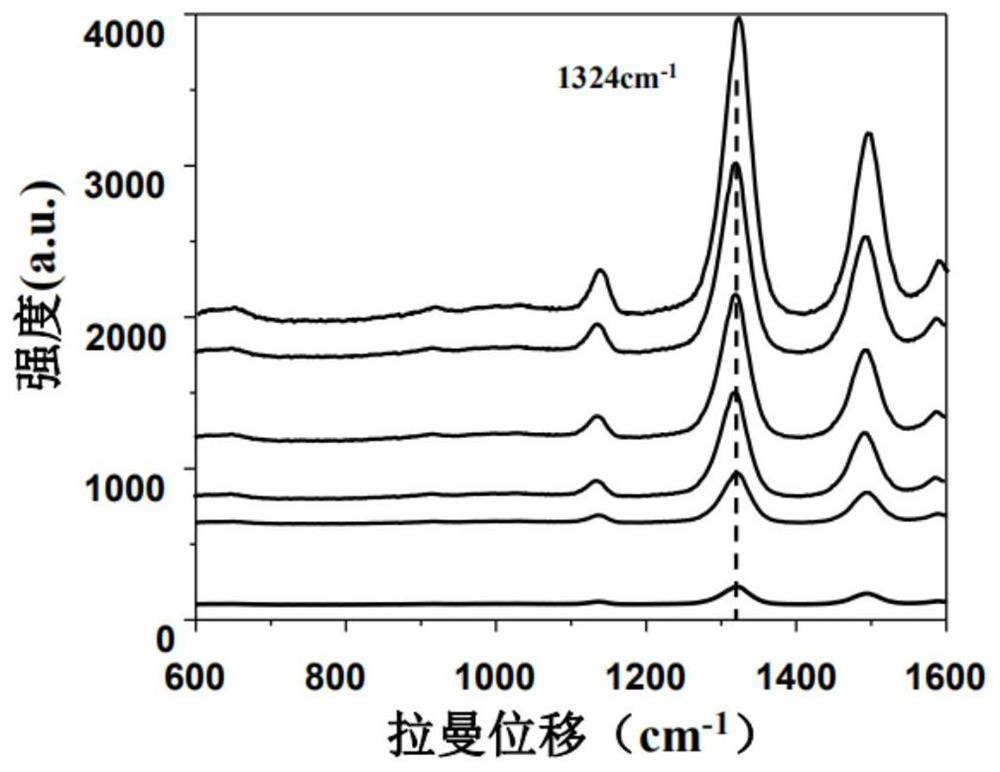
PendingCN113820481ARapid determinationEasy to operateRaman scatteringDisease diagnosisAnticoagulation AgentsCreatine kinase isoenzymes
The invention discloses a sandwich immunoassay kit. The invention provides an application of a surface-enhanced Raman detection kit in creatine kinase isoenzyme detection, the kit comprises a top layer reagent, a bottom layer reagent, a BSA solution and an EDTA anticoagulant, the top layer reagent in the kit adopts 3-mercapto-1, 2, 4-triazole molecules as Raman detection molecules, and compared with common P-ATP molecules in the prior art, the 3-mercapto-1, 2, 4-triazole molecules have a stronger Raman signal enhancing function; the surface-enhanced Raman detection kit is applied to the detection of creatine kinase isoenzyme in a human body for the first time, can make up for the defects in the existing detection technology, greatly improves the detection limit and sensitivity, and plays a key role in preventing sepsis.
Owner:BOZHOU CITY THE NEW HEALTH TECH CO LTD
High-sensitivity creatine kinase isozyme quantitative detection test strip
PendingCN111141905AHigh detection sensitivityEasy to holdChemiluminescene/bioluminescenceDisease diagnosisAcridineStreptavidin
The invention discloses a high-sensitivity creatine kinase isoenzyme quantitative detection test strip which comprises a connecting plate and a reagent tube integrally formed with the connecting plate, the connecting plate is sequentially divided into a handheld area, a reagent area, a washing area and a detection area, antiskid lines are arranged on an upper surface and a lower surface of the handheld area, and a sample reagent tube and a main reagent tube are arranged in the reagent area; a streptavidin magnetic particle solution, a biotin-labeled creatine kinase isoenzyme antibody solution,an acridinium ester-labeled creatine kinase isoenzyme antibody solution, an acidic excitation solution and an alkaline excitation solution are respectively arranged in the main reagent tube; the principle of a double-antibody sandwich method is adopted, meanwhile, an avidin-biotin system is utilized, acridinium ester chemiluminiscence is adopted, the detection sensitivity of the test strip is greatly improved, the reaction time is shortened, and the reagent cost is reduced.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
A kit for specific detection of creatine kinase isozyme
ActiveCN113189337BHigh sensitivityHigh detection specificityDisease diagnosisBiological testingAntiendomysial antibodiesActive agent
Owner:BEIJING DIAGREAT BIOTECH CO LTD
A creatine kinase isoenzyme double reagent and preparation method thereof
ActiveCN107641642BGood storage stabilityThe deviation of the measured value is smallMicrobiological testing/measurementPhosphoric Acid EstersAntiendomysial antibodies
The invention mainly aims at providing a creatine kinase isozyme bi-reagent and a preparation method thereof. The reagent I is prepared from the following components: 90 to 120mmol / L of imidazole buffer solution, 15 to 25mmol / L of N-acetylcysteine, 25 to 35mmol / L of phosphocreatine, 1 to 10mmol / L of adenosine diphosphate, 1 to 10mmol / L of triphosadenine, greater than 1.5KU / L of glucose-6-phosphatedehydrogenase, greater than 2.5KU / L of heterophosphatase and greater than 2.0KU / L of creatine kinase isozyme antibody; 0.01 to 0.05 percent by mass concentration of polyvinylpyrrolidone, 0.01 to 0.05percent by mass concentration of tris(nonylphenyl) phosphate, 0.01 to 0.02 percent by mass concentration of lauryl dihydroxyethyl amine oxide, 0.05 percent of isomeric sodium ascorbate and the balance of water; the reagent II is prepared from the following components: by adopting a reagent II solution as a basis, 1 to 5mmol / L of nicotinamide adenine dinucleotide phosphate, 10 to 30mmol / L of glucose and the balance of water. In the case of storing for 14 days at 37 DEG C, the reagent stability is still relatively high, and measurement value deviation of the reagent is less than 10 percent.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
A kind of creatine kinase isoenzyme detection reagent
ActiveCN104374925BHigh activityEfficient removalMicrobiological testing/measurementMaterial analysisSodium acetateAntiendomysial antibodies
The invention discloses a creatine kinase isozyme detection reagent. The reagent is composed of reagent R1 and reagent R2 at a ratio of 4:1 by volume, wherein reagent R1 is composed of imidazole buffer, glucose, nanoparticles, N-acetylcysteine, sodium edetate, Adenosine diphosphate, coenzyme Ⅰ, ribonucleotide, pyruvate decarboxylase, 6-phosphate glucose dehydrogenase, hexokinase, goat anti-human CK-M polyclonal antibody and trehalose, reagent R2 is composed of imidazole buffer, creatine phosphate, sodium dodecylbenzenesulfonate and preservatives. The reagent of the invention is a stable, accurate and strong anti-interference reagent for detecting creatine kinase isoenzymes, and has high clinical application value.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Creatine Kinase Isoenzyme Assay Kit
ActiveCN113030470BStrong specificityGood repeatabilityMaterial analysisGlycineAntiendomysial antibodies
The invention discloses a creatine kinase isoenzyme (CKMB) assay kit, comprising a first reagent and a second reagent, wherein the first reagent is a phosphate buffer containing polyethylene glycol and heparin; the second reagent is a phosphate buffer containing polyethylene glycol and heparin; Creatine Kinase Isoenzyme Latex Antibody and Hyaluronic Acid in Glycine Buffer. The creatine kinase isoenzyme assay kit of the present invention can quantitatively and objectively reflect the existence of CKMB, and provide more powerful experimental diagnosis basis for clinical diagnosis, curative effect observation and prognosis judgment. The creatine kinase isozyme assay kit provided by the invention has high specificity, good repeatability, high detection sensitivity, wide linear range and high stability.
Owner:江西英大生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com