Screening kit and method for progressive muscular dystrophy of newborns
A technology for muscular dystrophy and neonates, which can be used in biomaterial analysis, instruments, measuring devices, etc., and can solve the problems of false positive results, inconsistency, and difficulty in popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
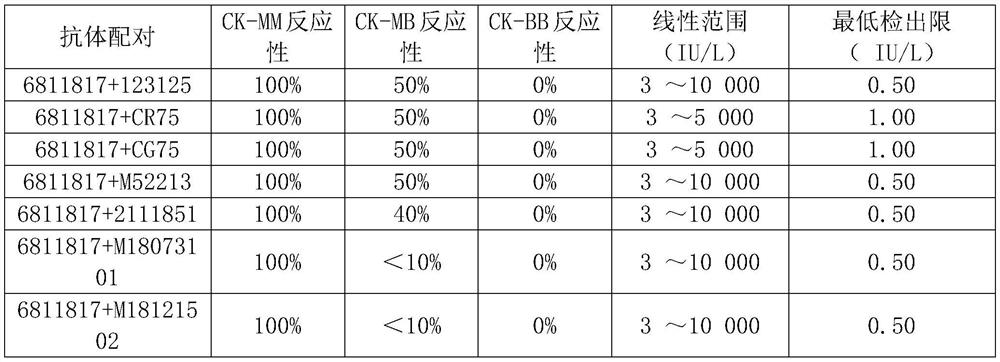
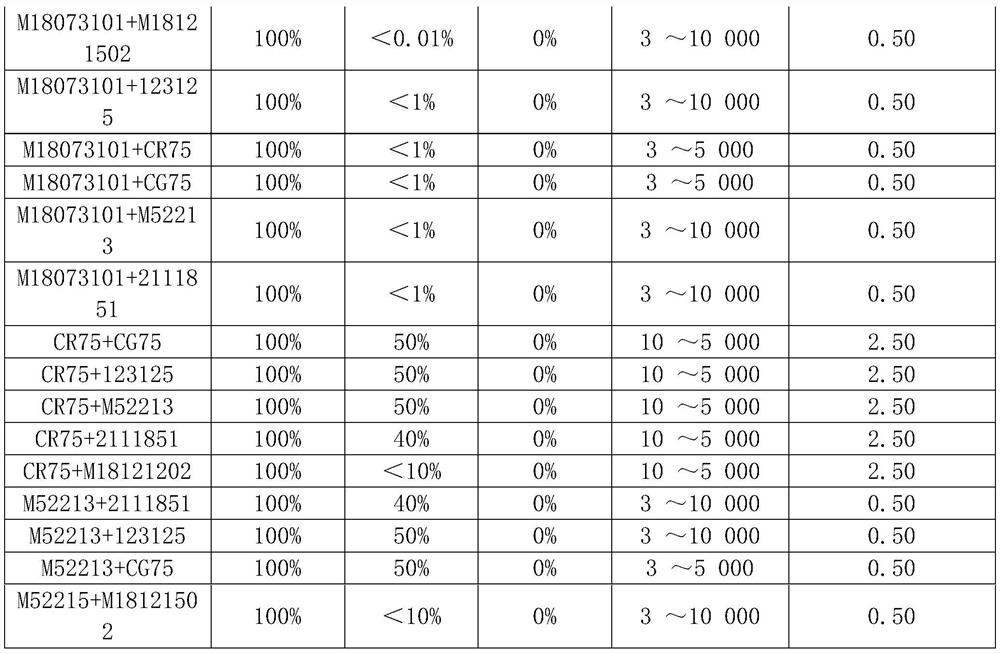
[0086] Antibody pairing detection effect of a creatine kinase isoenzyme (CK-MM) assay reagent (time-resolved fluorescent immunoassay) suitable for neonatal progressive muscular dystrophy screening:
[0087]
[0088]
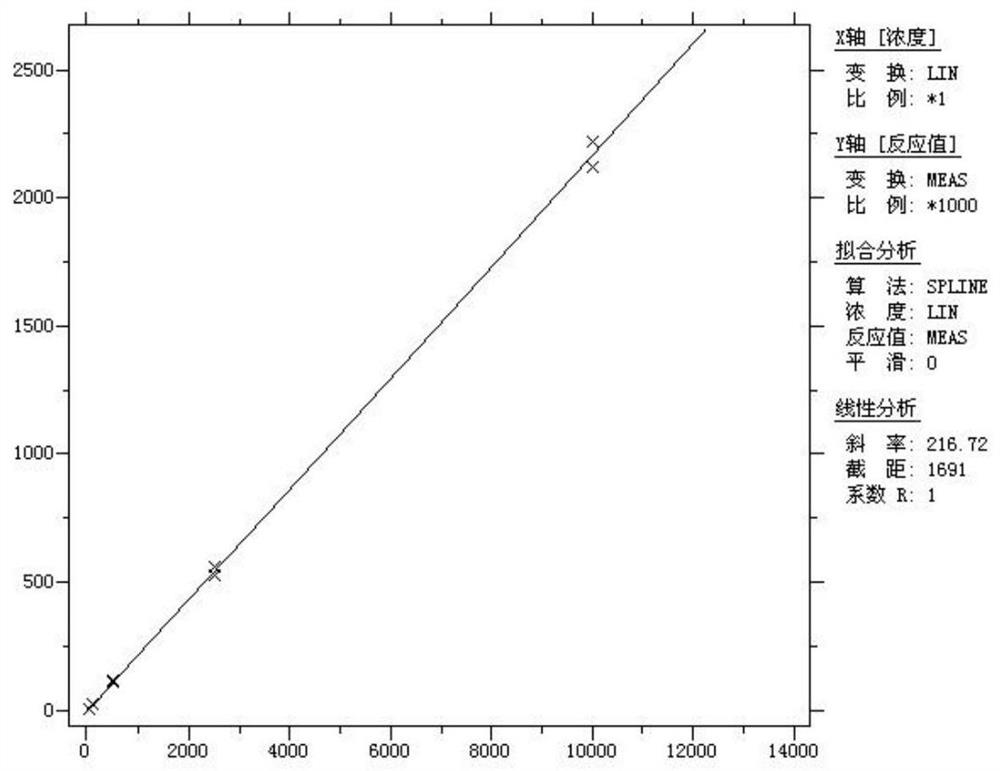
[0089] From the above results, the mouse monoclonal antibody M18073101 is paired with M18121502, which has 100% reactivity with CK-MM and strong sensitivity; low reactivity with CK-MB and CK-BB, strong specificity and wide linear range.
Embodiment 2
[0091] A creatine kinase isoenzyme (CK-MM) assay reagent (time-resolved fluorescent immunoassay) suitable for neonatal progressive muscular dystrophy screening includes:
[0092] Capture antibody solid phase carrier: coated with human skeletal muscle CK-MM monoclonal antibody 5μg / mL;
[0093] Calibrator: The filter paper dry blood film calibration product contains 6 concentration points of A, B, C, D, E, F, calibrator A is zero concentration, and calibrator B, C, D, E, F contains muscle with a series of concentration gradients. Acid phosphokinase; the calibrator was traced to the standard substance GBW09167. The concentrations of A, B, C, D, E, and F were 0, 20, 100, 500, 2500, and 10 000 IU / mL, respectively.
[0094] Detection of antibody markers: Europium-labeled human skeletal muscle CK-MM monoclonal antibody.
[0095] Sample eluent: 50mmol / L, tris-hydrochloric acid buffer containing 0.02% (v / v) of pH 7.8 20ppm thimerosal, 30% (v / v) SignalBoostTM immune signal enhancer,...
Embodiment 3
[0100] The specific operations of the creatine kinase isoenzyme (CK-MM) assay reagent (time-resolved fluorescent immunoassay) of the above embodiment 2 are as follows:
[0101] 1. Reagent Preparation
[0102] 1) Capture antibody solid-phase carrier: Equilibrate the reagent and the required amount of capture antibody solid-phase carrier to room temperature (20-25°C). The rest of the capture antibody solid-phase carrier was placed in a ziplock bag in time to be sealed and stored at 2-8°C.
[0103] 2) Cleaning working solution: Add cleaning solution and purified water at a ratio of 1:2 to a clean container and mix them as a cleaning working solution.
[0104] 3) Detection antibody marker working solution: Prepare within 30 minutes before use, add the detection antibody marker and experimental buffer at a ratio of 1:1 200 to a clean disposable container and mix well; use it up for the current experiment.
[0105] 2. Detection steps
[0106] 1) Use the automatic nail punching me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com