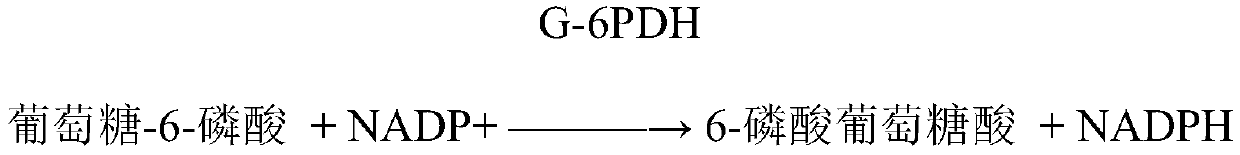Phosphocreatine kinase isoenzyme detection kit
A detection kit, creatine phosphate technology, applied in the field of biomedicine, can solve the problem of low sensitivity, achieve high sensitivity, good stability, and meet the actual clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0027] 1. Main materials and instruments
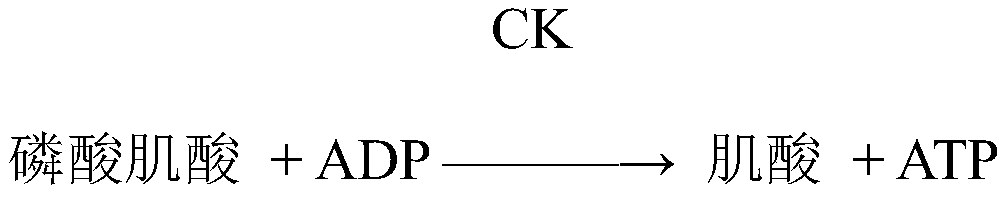
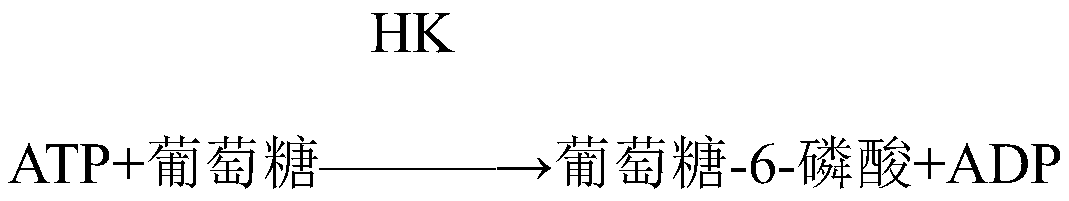
[0028] First reagent: imidazole, magnesium acetate, ethylenediaminetetraacetic acid, mannitol, adenosine monophosphate, lithium diadenosine pentaphosphate, reduced coenzyme II, Proclin150, hexokinase, N-acetylcysteine, sodium sulfite , anti-human CK-M antibody.
[0029] The second reagent: phosphate buffer saline, Proclin150, creatine phosphate, glucose, 5'-adenosine diphosphate disodium salt, bovine serum albumin, glucose-6-phosphate dehydrogenase, gentamicin.
[0030] Instrument: AL104 precision electronic balance: sensitivity 0.1mg, used for weighing process;
[0031] TC30K precision electronic balance: sensitivity 10mg, used for weighing process;
[0032] T1000Y electronic balance: sensitivity 10mg, used for weighing process;
[0033] Magnetic stirrer: Type 99-1, used for dissolution process.
[0034] Precision acidity meter: FE20, used in PH process.
[0035] Electronic scale: sensitivity 20g, used for constant volume proces...
Embodiment
[0051] 3. Performance test
[0052] 3.1 Batch number and specifications of creatine phosphokinase isoenzyme detection kit (CKMB kit for short)
[0053] Different batches of the kits in the aforementioned preparation examples were made respectively, named according to the batches, namely sample 1, sample 2, and sample 3. There are 6 packaging specifications for this product, but the size and packaging are different, which has no effect on the performance evaluation results , so only one of the most commonly used specifications was tested, and the specification was 50ml (the first reagent: 1×40ml, the second reagent: 1×10ml). The first reagent and the second reagent are transparent liquids without precipitation and floc.
[0054] 3.2 Calibrators and quality controls
[0055] Calibrator for performance evaluation: Calibration with factor, K factor value 8360;
[0056] Reference material for accuracy performance evaluation: Japan Toyobo, Lot: J234, marked value: 179U / L;
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com