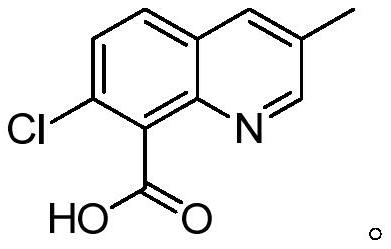
Quinmerac and preparation method thereof
A technology of quinolinic acid and chloroquine, which is applied in the direction of organic chemistry, can solve the problems of difficult industrialization of waste acid and waste water, and achieve the effects of less side reactions, lower treatment costs of three wastes, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
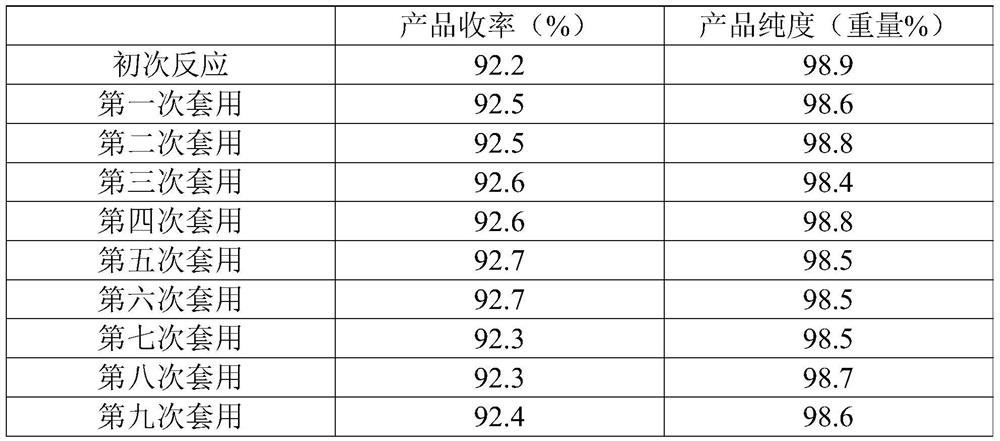
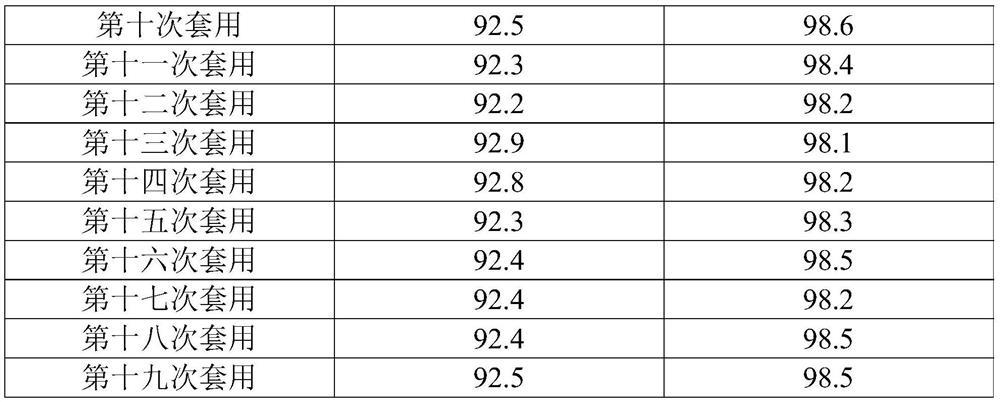
[0042]1) In a 2L four-neck glass flask, add 200 grams of 3,8-dimethyl-7-chloroquinoline, 800 grams of acetic acid, 9.26 grams of cobalt acetate (0.05 equivalents), 2.7 grams of sodium bromide (0.025 equivalents), and heat up To 100°C, wait until all the solids are dissolved, then feed the mixed gas of ozone and air at 0.4L / min, and detect the reaction in the liquid phase. After reacting for 12 hours, the temperature was lowered to 25° C., the solid was precipitated, filtered, rinsed with 30 g of acetic acid, the solid was dried, and the filtrate was used for the next batch of reactions. 214.7 g of quinclorac solid was obtained (NMR data as follows), with a purity of 98.9% by weight and a yield of 91.8%. After filtering the mother liquor, the corresponding 3,8-dimethyl-7-chloroquinoline was added, and the reaction was continued.
[0043] 1 H-NMR (500MHz, d6-DMSO) δ: 8.819(s, 1H), 8.128(s, 1H), 7.761-7.743(d, 1H, J=9Hz), 7.581-7.563(d, 1H, J=9Hz ), 2.763(s,3H), 2.497(s,3H). ...
Embodiment 2
[0049] 1) In a 2L four-necked glass flask, add 200 grams of 3,8-dimethyl-7-chloroquinoline, 1000 grams of acetic acid, 14 grams of manganese acetate (0.05 equivalents), 2.7 grams of sodium bromide (0.025 equivalents), and heat up To 100°C, wait until all the solids are dissolved, then feed the mixed gas of ozone and air at 0.4L / min, and detect the reaction in the liquid phase. After reacting for 12 hours, the temperature was lowered to 25° C., the solid was precipitated, filtered, rinsed with 50 g of acetic acid, the solid was dried, and the filtrate was used for the next batch of reactions. 215.8 grams of quinclorac solid were obtained, with a purity of 98.9% by weight and a yield of 92.2%. After filtering the mother liquor, the corresponding 3,8-dimethyl-7-chloroquinoline was added, and the reaction was continued.
[0050] The first application: In a 2L four-neck glass flask, add 200 grams of 3,8-dimethyl-7-chloroquinoline, 1010.4 grams of filtrate, heat up to 100 ° C, wait...
Embodiment 3
[0056] 1) In a 2L four-neck glass flask, add 200 grams of 3,8-dimethyl-7-chloroquinoline, 1000 grams of acetic acid, 9.3 grams of vanadium pentoxide (0.05 equivalents), and 2.7 grams of sodium bromide (0.025 equivalents) , the temperature was raised to 80°C, and when all the solids were dissolved, a mixed gas of ozone and air was introduced at 0.6 L / min, and the reaction was detected in the liquid phase. After reacting for 12 hours, the temperature was lowered to 25° C., the solid was precipitated, filtered, rinsed with 50 g of acetic acid, the solid was dried, and the filtrate was used for the next batch of reactions. 214 grams of quinclorac solid were obtained, with a purity of 98.7% by weight and a yield of 91.3%. After filtering the mother liquor, the corresponding 3,8-dimethyl-7-chloroquinoline was added, and the reaction was continued.
[0057] The first application: In a 2L four-neck glass flask, add 200 grams of 3,8-dimethyl-7-chloroquinoline, 1008.8 grams of filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com