A kind of green synthesis method of high-purity tasteride
A dutasteride and green technology, applied in the field of pharmaceuticals, can solve problems such as difficult complete recovery, and achieve the effect of improving purity and refining efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
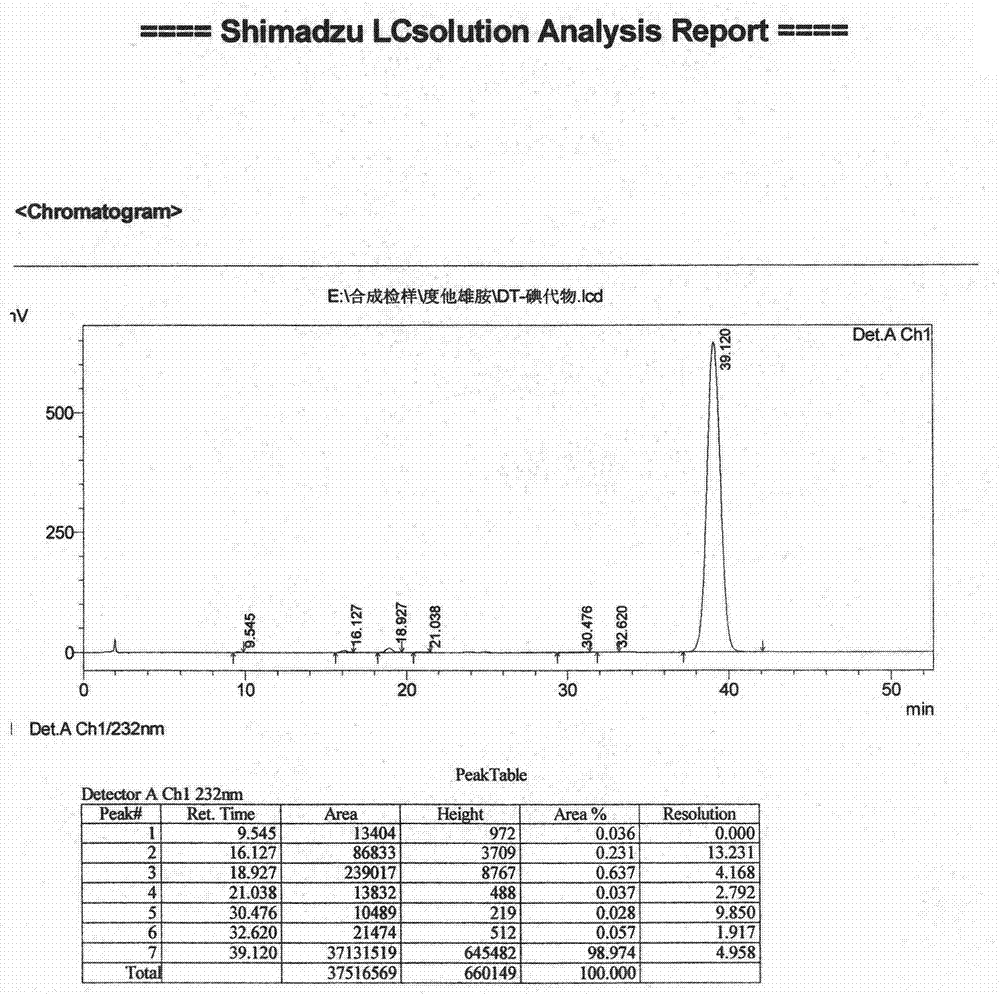
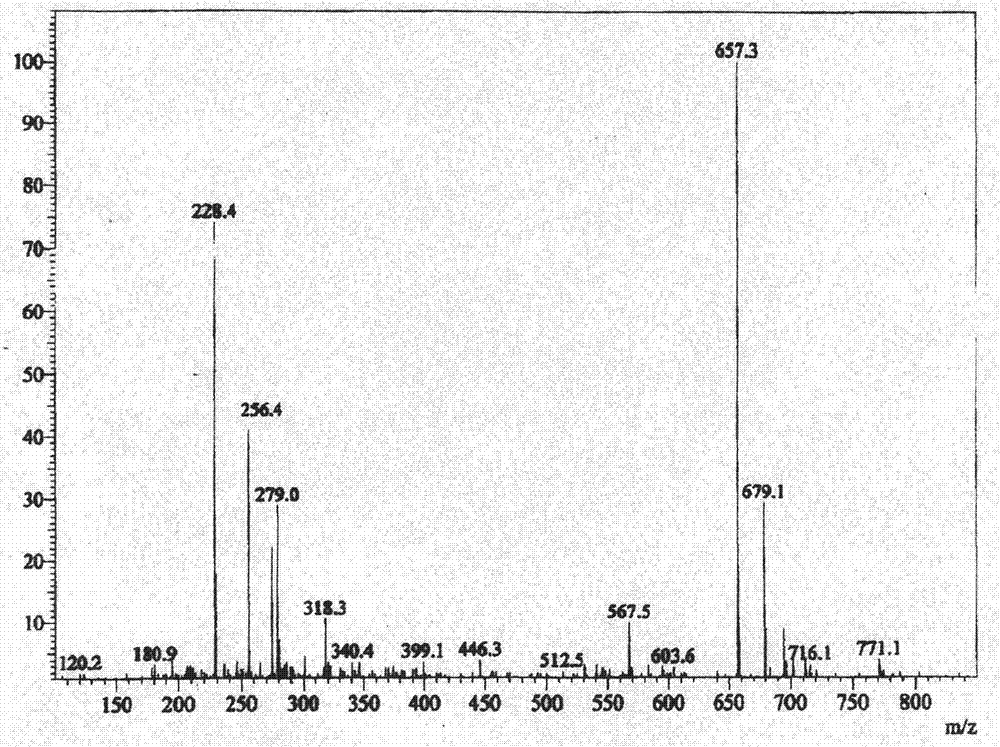
[0046] Embodiment 1 (preparation of iodide)
[0047] Add (5α, 17β)-N-[2,5-bis(trifluoromethyl)phenyl]-3-oxo-4-azandrost-17-carboxamide (amination) 3.56 in a 200-liter reactor Kg and dichloromethane 110Kg, stir, add 3.12Kg of tetramethylethylenediamine under cooling, continue to stir and cool down to -10°C, add 2.2Kg of trimethylchlorosilane dropwise, slowly warm up to room temperature and stir for 2 hours. Cool down to -20~-15°C, add 2.9Kg of iodine in batches, and keep warm until the reaction is complete. Under cooling, add 10% sodium bisulfite solution to quench the reaction, let stand to separate the layers, extract the water layer with dichloromethane, combine the organic phases and wash with 5% hydrochloric acid and purified water successively until neutral, anhydrous Dry over sodium sulfate and concentrate to dryness under reduced pressure. The resulting residue was added to acetonitrile, beaten at room temperature, cooled and crystallized, filtered, and dried under re...
Embodiment 2
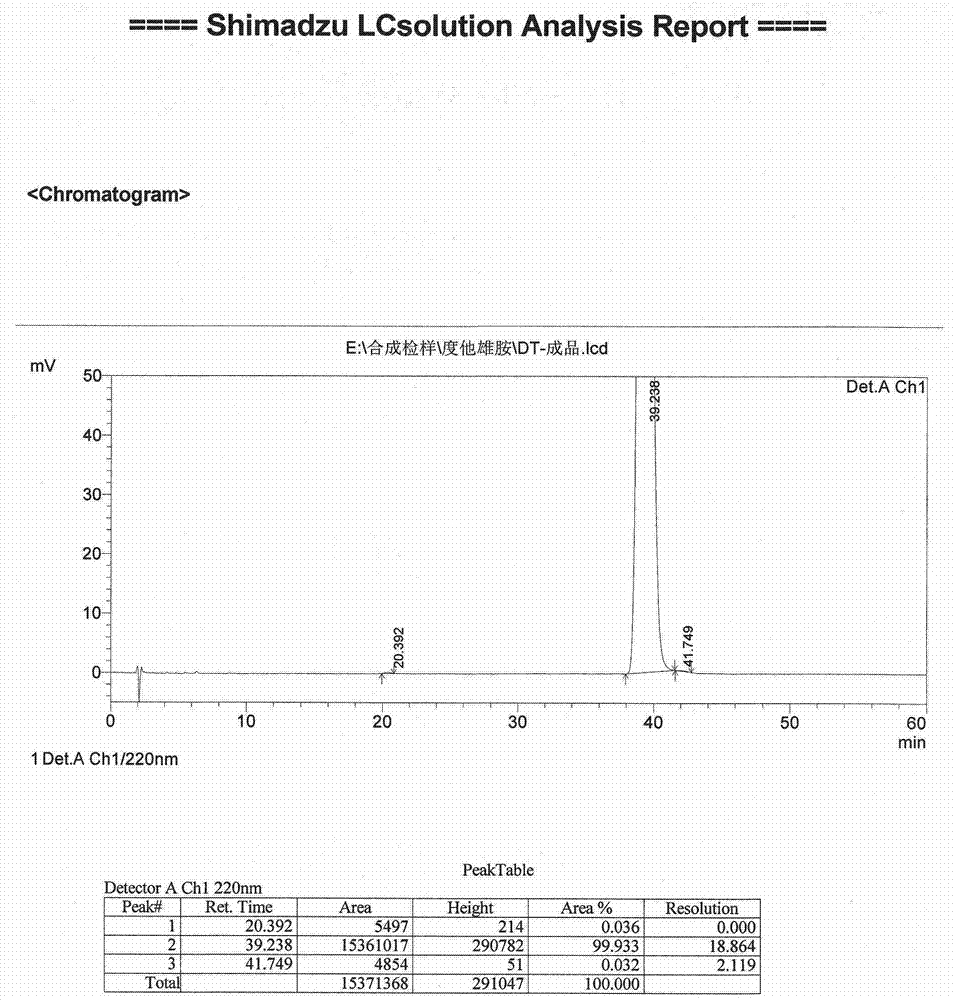
[0050] Embodiment 2 (preparation of dutasteride)
[0051] Put 6.84Kg of potassium tert-butoxide and 60Kg of N,N-dimethylformamide (DMF) into a 200-liter reactor, stir to dissolve, lower the temperature to -20~-10°C, and add the compound prepared in Example 1 dropwise. The mixed solution of iodide 4.00Kg and DMF20Kg, and insulation reaction to complete. After cooling, add acetic acid dropwise to quench the reaction, add sodium chloride solution for crystallization, filter and dry to obtain crude dutasteride.
[0052] Dissolve the above crude product in dichloromethane, wash with potassium carbonate solution and saturated sodium chloride solution until neutral, concentrate to dryness under reduced pressure, add acetonitrile to dissolve the obtained concentrate, add 2.5Kg hydrochloric acid to stir crystallization, filter, Wash with purified water, add dichloromethane to redissolve the filter cake, wash with purified water until neutral, separate liquids, stir and dry the obtaine...
Embodiment 3
[0053] Embodiment 3 (small scale preparation of iodide)
[0054] Add 300g of amide, 230g of triethylamine and 6L of dichloromethane into a 20-liter reactor, stir, cool down to 0°C, control the temperature at -25°C to 15°C, add 185g of trimethylchlorosilane dropwise, complete the addition, and raise the temperature to 15°C ℃ ~ 35 ℃ stirring reaction is complete, then lower the temperature to -25 ℃, add 200g of iodine in batches, and react at -15 ℃ ~ 0 ℃. After the reaction was completed, sodium thiosulfate solution was added to quench the reaction, and the liquid was separated after standing. The organic phase was washed with dilute hydrochloric acid and purified water successively, dried over anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure. The resulting residue was added into acetonitrile, crystallized, filtered, and dried under reduced pressure to constant weight to obtain 347 g of iodide, yield 91%, purity: 98.6%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com