Chitosan oligosaccharide-g-citronellol derivative and preparation method thereof
A technology of oligochitosan and citronellol, which is applied in the field of oligochitosan-g-citronellol derivatives and its preparation, can solve the problems of low anti-inflammatory activity and very limited research on anti-inflammatory activity, and achieve foot swelling Good, good water solubility, wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
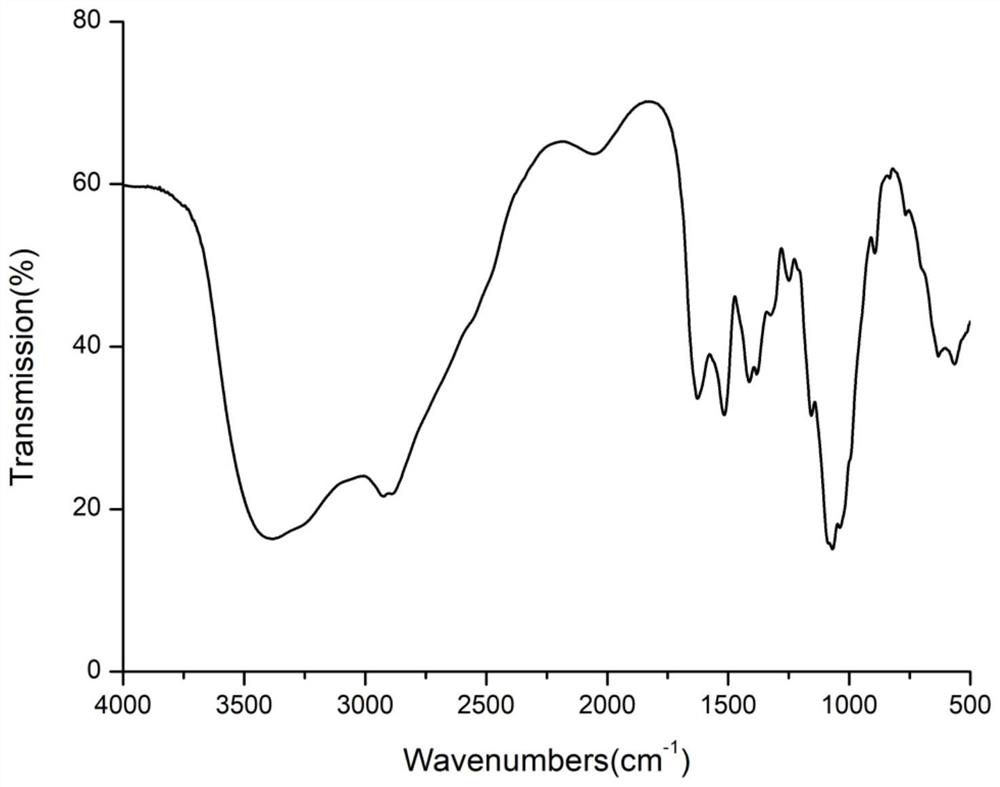
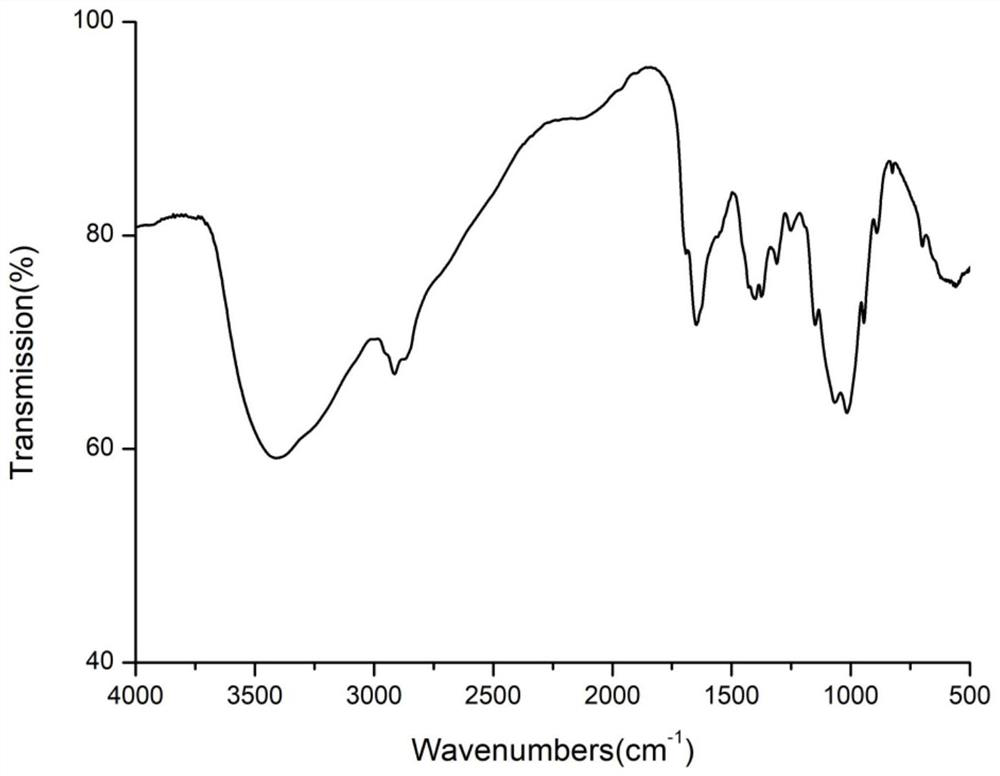
Image
Examples
Embodiment 1
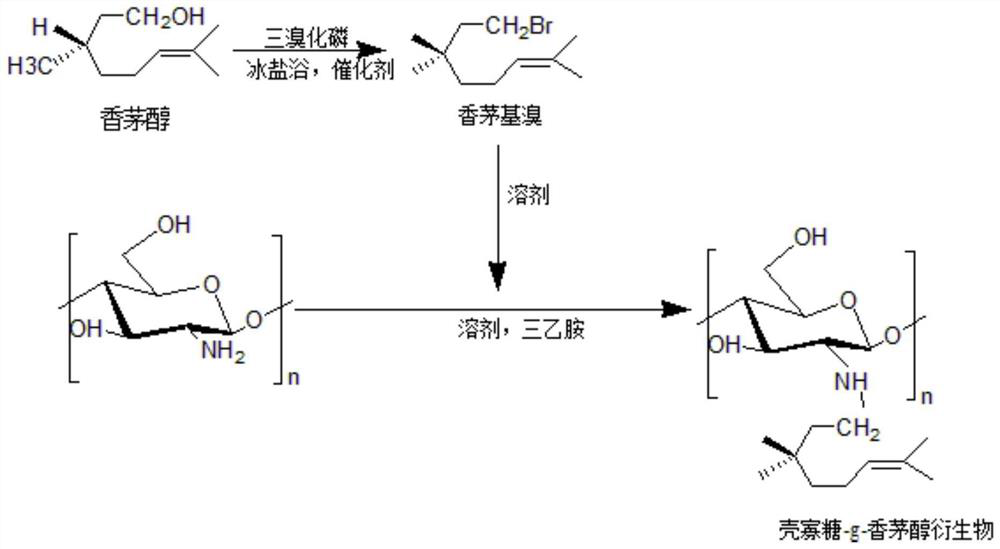
[0033] Embodiment 1: the preparation of chitosan oligosaccharide-g-citronellol derivative
[0034] 1. Preparation of Citronellyl Bromide
[0035] Dissolve 5mL of citronellol and 2mL of phosphorus tribromide in 30mL of anhydrous ether respectively, add chitosan oligosaccharide solution and 0.45mL of pyridine into a three-neck flask, stir rapidly under ice-salt bath conditions, and then dissolve the tribromide Phosphorus solution was slowly added dropwise to the above reaction system. After the dropwise addition was completed, continue to stir rapidly for 45 minutes, then washed with 5% sodium bicarbonate solution, deionized water and saturated saline for 3 times, and dried by adding anhydrous magnesium sulfate. Filtrate with a 0.22um filter membrane, concentrate by rotary evaporation at 35°C for 30min to obtain a light yellow oily liquid citronellyl bromide.
[0036] 2. Preparation of oligochitosan-g-citronellol derivatives
[0037] After dissolving 1g of chitosan oligosaccha...
Embodiment 2
[0043] Embodiment 2: the preparation of chitosan oligosaccharide-g-citronellol derivative
[0044] 1. Preparation of Citronellyl Bromide
[0045] Dissolve 5mL of citronellol and 3mL of phosphorus tribromide in 40mL of anhydrous ether respectively, add chitosan oligosaccharide solution and 0.5mL of pyridine into a three-neck flask, stir rapidly under ice-salt bath conditions, and then add tribromide Phosphate solution was added dropwise to the above reaction system. After the dropwise addition, continued to stir rapidly for 90 min, then washed 4 times with 5% sodium bicarbonate solution, deionized water and saturated brine, and dried by adding anhydrous magnesium sulfate. Filter with a 0.22um filter membrane, and concentrate by rotary evaporation at 40°C for 40min to obtain a light yellow oily liquid citronellyl bromide.
[0046] 2. Preparation of oligochitosan-g-citronellol derivatives
[0047] After dissolving 1g chitosan oligosaccharide and 4mL citronellyl bromide with dim...
Embodiment 3
[0048] Embodiment 3: the preparation of chitosan oligosaccharide-g-citronellol derivative
[0049] 1. Preparation of Citronellyl Bromide
[0050] Dissolve 5mL of citronellol and 4mL of phosphorus tribromide in 50mL of anhydrous ether respectively, add chitosan oligosaccharide solution and 0.55mL of pyridine into a three-neck flask, stir rapidly under ice-salt bath conditions, and then dissolve the tribromide Phosphorus solution was added dropwise to the above reaction system. After the dropwise addition was completed, the rapid stirring was continued for 120 min, followed by washing with 5% sodium bicarbonate solution, deionized water and saturated brine for 5 times, adding anhydrous magnesium sulfate to dry, and using Filter through a 0.22um filter membrane, and concentrate by rotary evaporation at 50°C for 45min to obtain a light yellow oily liquid citronellyl bromide.
[0051] 2. Preparation of oligochitosan-g-citronellol derivatives
[0052] Dissolve 1g of oligochitosan ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com