Preparation method of (S)-ketamine hydrochloride as well as intermediate and crystal form thereof
A technology of ketamine and crystal form, which is applied in the preparation of carboxylate, the preparation of carboxylate, the preparation of organic compounds, etc., can solve problems such as low yield, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 2-(2-chlorophenyl)-2-methylaminocyclohexanone-L-(+)-tartrate dihydrate
[0049] Accurately weigh 1.0 g of ketamine racemate in a 25 mL three-neck flask, add 9.4 mL of acetone, dissolve the solid, heat and stir in an oil bath at 65°C, and add the prepared L-tartaric acid aqueous solution (L- Tartaric acid 0.347g and water 0.625mL), the solution became turbid about 10-30min after adding, kept stirring at this temperature for 1h, then gradually lowered the temperature, and kept stirring for 5-6h after cooling down to room temperature, suction filtered the obtained solid, and used for filter cake 10 mL of acetone was washed three times, and the resulting white solid was vacuum-dried at 40°C for 2-3 hours, and weighed to obtain 0.74 g, with a yield of 41.5%, a chiral purity of 99.72%, and an ee value of 99.5%.
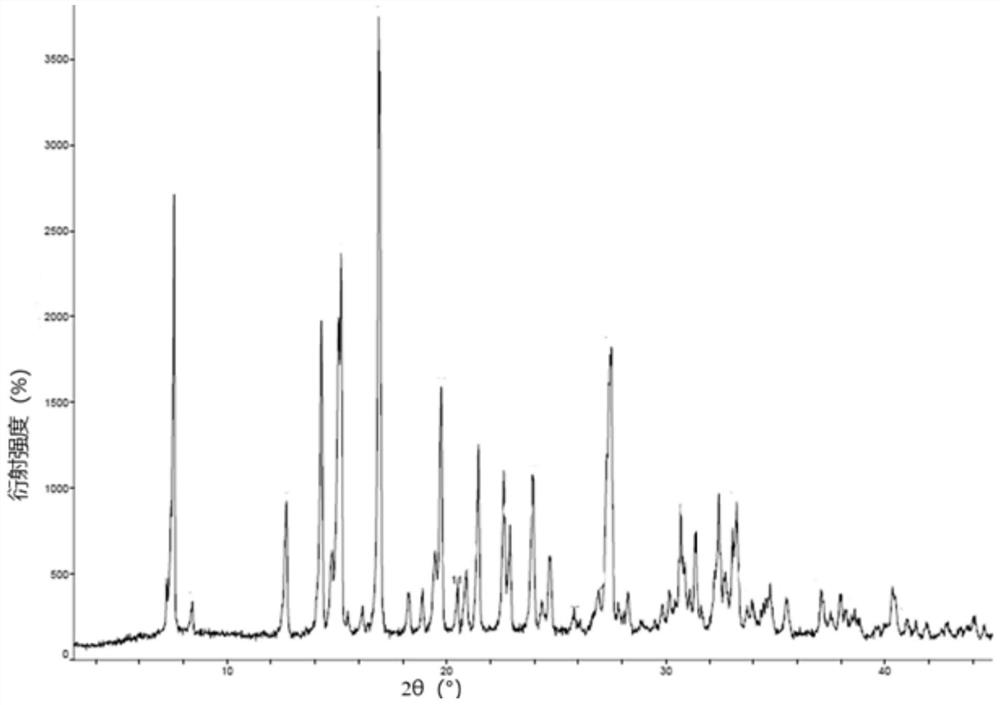
[0050] The PXRD diffractogram of the product, and a representative example such as figure 1 shown.
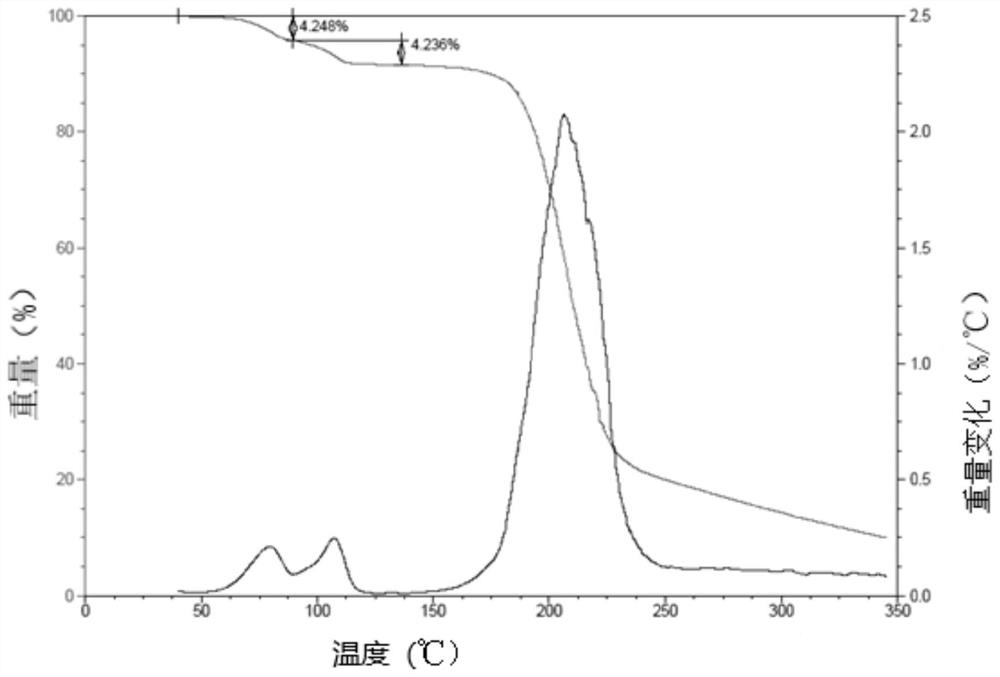
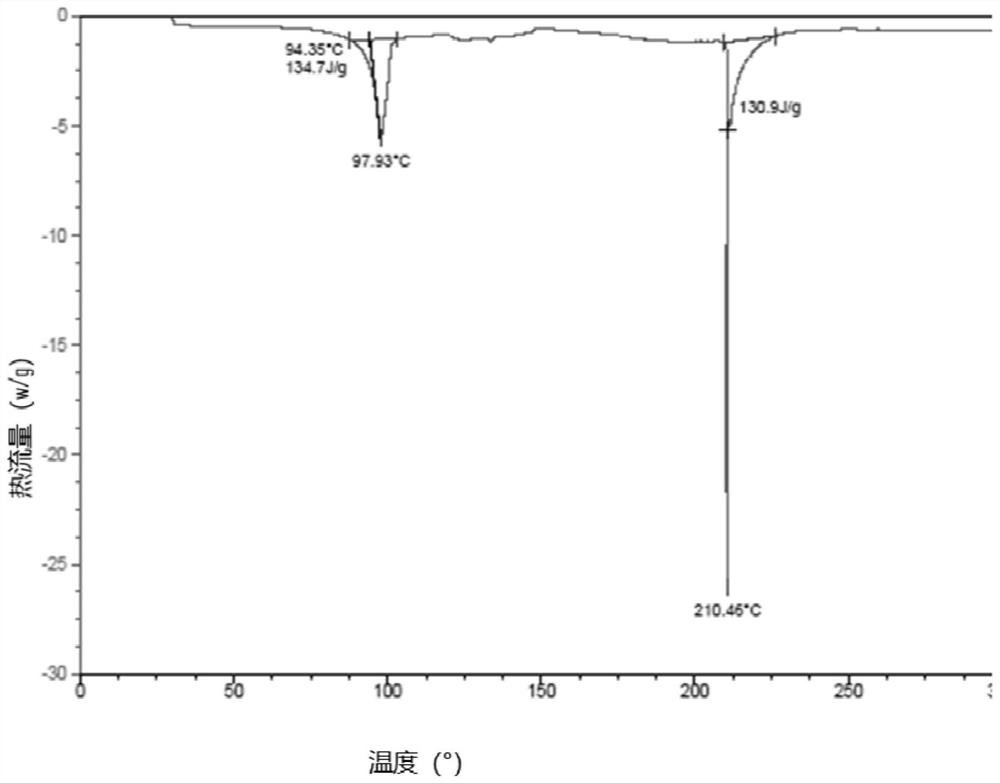
[0051] TGA measurements on a representative sample o...
Embodiment 2
[0060] Example 2 Synthesis of 2-(2-chlorophenyl)-2-methylaminocyclohexanone hydrochloride
[0061] Accurately weigh 0.7g of S-ketamine-L-tartrate into a 25mL single-necked bottle, add 7mL of water to dissolve it, then slowly add 5mL of 1mol / L sodium hydroxide aqueous solution dropwise, gradually precipitate solids, adjust to pH=10-11, Stir for 30 min, filter with suction, wash the solid with water, then dry it in vacuum at 60°C for 6-10 h, add the obtained dry solid (0.4 g) into a one-necked flask, dissolve it with 3 mL of ethyl acetate, then slowly add EA-HCl ( 3mL), a white solid was gradually precipitated, then stirred at room temperature for 2h, suction filtered, ethyl acetate (10mL) washed the filter cake three times, the resulting white solid was vacuum-dried at 40°C for 6-8h, and weighed to obtain 0.44g, with a yield of 97.8%. The chiral purity is 99.9%, and the ee value is 99.9%. The test conditions of ee value are shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com