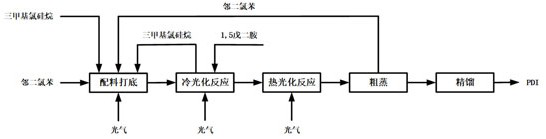
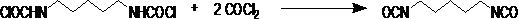Method for preparing 1,5-pentamethylene diisocyanate
A technology of pentamethylene diisocyanate and o-dichlorobenzene, applied in the field of 5-pentane diisocyanate, preparation 1, which can solve the problems of complex process, high technical difficulty, and influence on the yield of photochemical reaction PDI, so as to improve yield and product Quality, the effect of reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The principles and features of the present invention will be further elaborated below through examples, which are only used to explain the present invention and are not intended to limit the scope of the present invention.
[0022] The experimental conditions of the following examples are: the equipment used in the test is a 20L double-layer glass reactor; cold photochemical reaction temperature 15 ~ 30 ℃, reaction pressure ~ 0.2MPa, reaction time 4 ~ 6h; thermophotochemical reaction temperature 150 ℃ ~ 170°C, reaction pressure 0.2-0.3MPa, reaction time 8-12h.
[0023]
[0024] From the above examples, it can be obtained that under the same experimental conditions, the reaction result of the luminescence reaction with the addition of a catalyst, the generation of hydrochloride can be completely ignored, and the yield of dicarbamoyl chloride can reach more than 98.5%; the result of the thermophotochemistry reaction The yield of PDI products can reach more than 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com