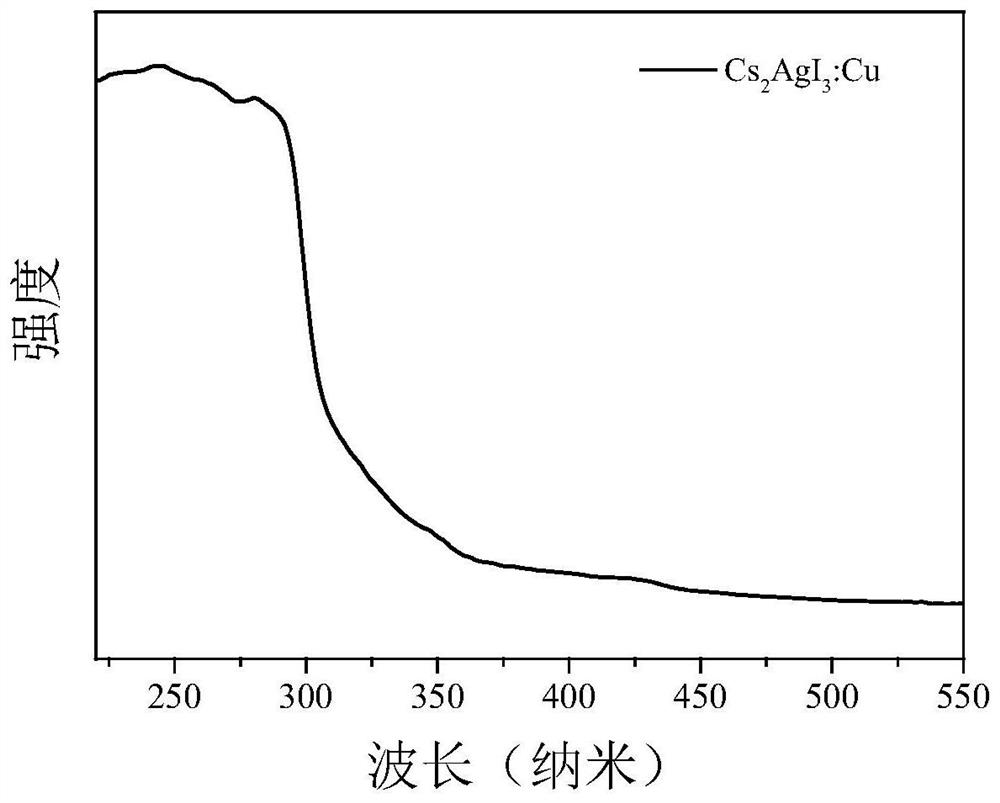
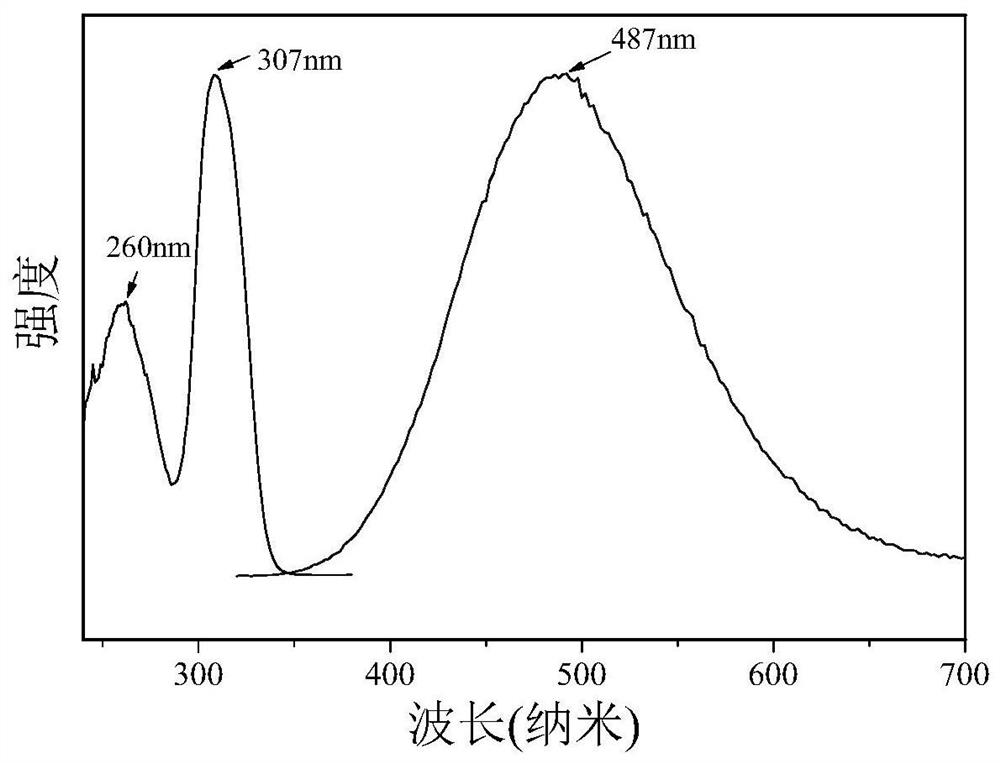
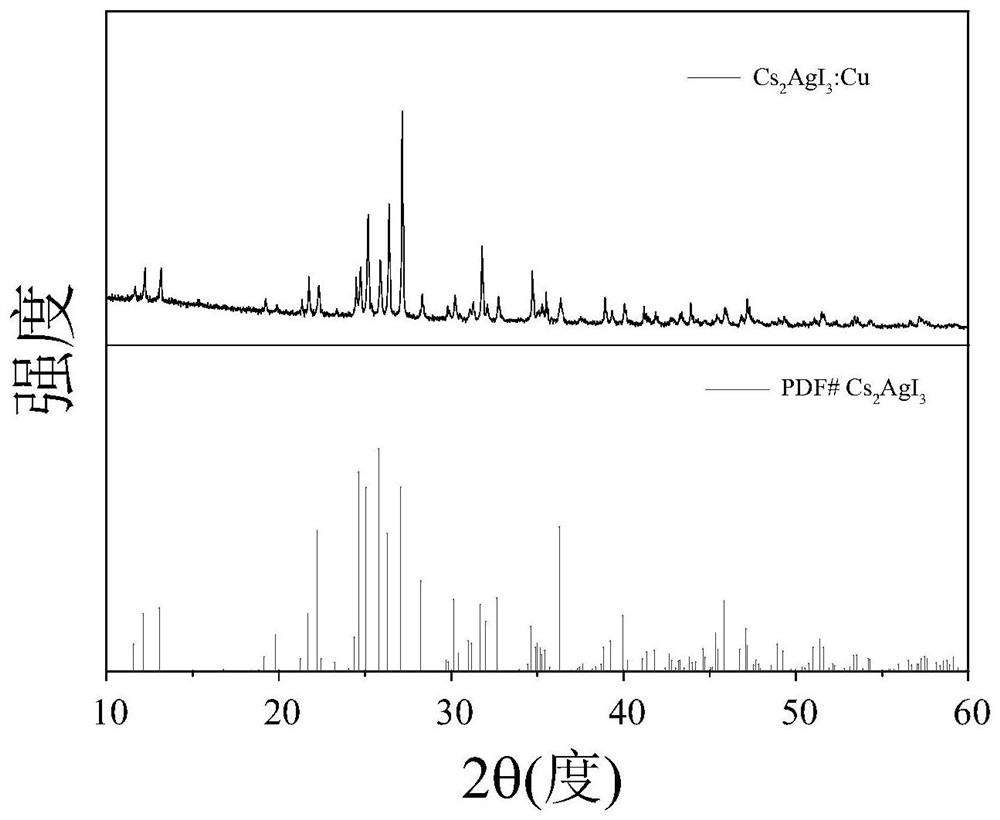
High-fluorescence-efficiency inorganic lead-free perovskite material and preparation method thereof
An inorganic non-perovskite technology, applied in the direction of luminescent materials, chemical instruments and methods, can solve the problems of high energy consumption, long time consumption, and lack of fluorescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 2mmol cesium iodide and 1mmol silver iodide in the glove box, pour them into a 25mL agate jar containing 25 agate balls with a diameter of 6mm, seal the agate jar with a sealing film, take it out of the glove box, set The AC frequency of the ball mill is 35Hz, and the corresponding rotating speed is 1050rad / min at this time. After mechanical grinding for 10 hours, the yellow-green mixture powder changes from fluffy to dense with the prolongation of the grinding time, and finally turns into a fluffy light yellow powder. Stop grinding. When irradiated with an ultraviolet lamp with an excitation wavelength of 302nm, the product has no fluorescence. Put the obtained product into a hydrothermal reaction kettle, add 0.2mol cuprous iodide, 30 microliters of hypophosphorous acid, and 2 milliliters of hydroiodic acid, maintain at 180°C for 5 hours, and cool down to room temperature through a 24h program. After annealing at room temperature, after cooling at -15 °C for 1.5 ...
Embodiment 2
[0026] In Example 1, due to Cu + Instability, it is very prone to disproportionation reaction to generate Cu 2+ and Cu, therefore hypophosphorous acid must be added, now the amount of hypophosphorous acid is changed from 30uL in Example 1 to 10uL, 20uL, 50uL respectively, other conditions and steps are unchanged, the fluorescence efficiency of each product is measured to be 59.4%, 62.8% respectively. %, 63.7%, so the optimal dosage of hypophosphorous acid is 30uL.
Embodiment 3
[0028] Change the hydroiodic acid consumption in embodiment 1 into 1mL, 1.5mL, 3mL respectively by 2mL, other conditions and steps are constant, record the fluorescence efficiency of each product to be respectively 50.4%, 54.6%, 47.1%, therefore hydroiodide The optimal acid volume is 2mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com