Mycoplasma ovipneumoniae vaccine strain, vaccine composition prepared from vaccine strain and application of vaccine composition
A technology of mycoplasma pneumoniae and vaccine composition, applied in vaccines, multivalent vaccines, veterinary vaccines, etc., can solve the problems of difficult isolation of mycoplasma pneumoniae, limited protection of sheep, and difficulty in differential diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
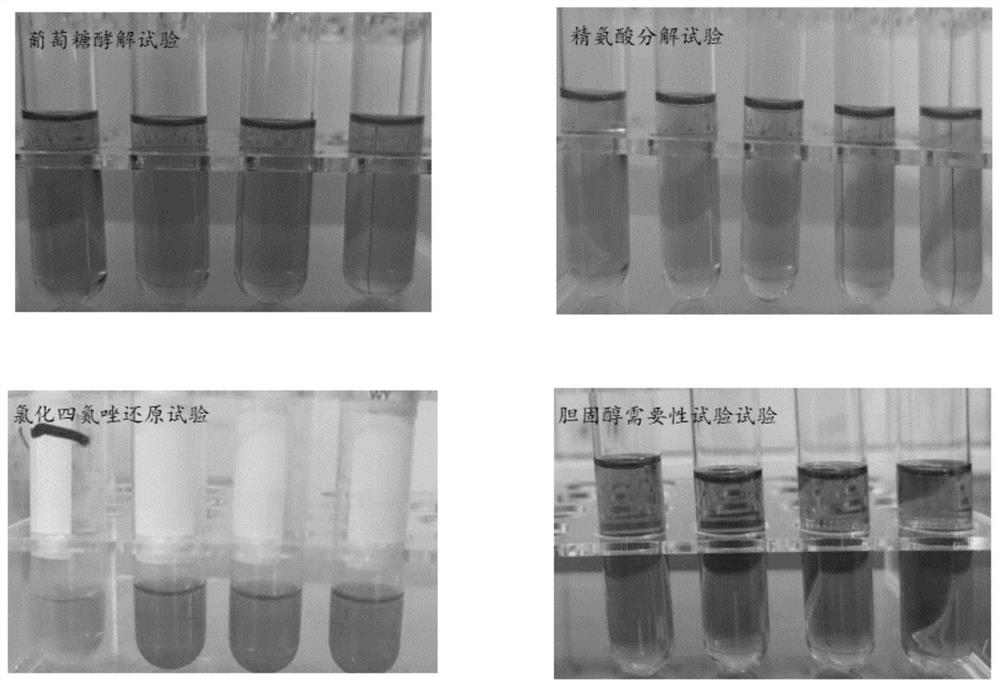
[0054] Example 1. Isolation and preservation of Mycoplasma ovis WM01 F6 strain
[0055] 1.1 Collection of disease materials
[0056] Tissue materials from sheep with respiratory diseases were collected. The collection site is the pleural cavity, lungs, pleural effusion, and nasal swabs of infected sheep. After opening the pleural cavity, the lungs and pleural effusion are quickly cut off the infected part of the lung with prepared sterilized scissors and placed in a sterilized plate. Use a sterile syringe The pleural effusion is extracted and placed in a sterile test tube. Use a swab to get the nasal fluid into a sterile tube. Place the above-mentioned disease materials in an insulated bucket and refrigerate them in the refrigerator. The disease material was transported back to the laboratory within 12 hours and stored in a refrigerator at 4°C. The blood of the sick sheep was collected in 2ml EP tubes and brought back to the laboratory at 2-8°C.
[0057] 1.2 Disease mater...
Embodiment 2
[0078] The pathogenicity test of embodiment 2 ovine mycoplasma pneumoniae WM01 F6 strain
[0079] The test selected 6 healthy susceptible sheep (negative for Mycoplasma ovis ELISA antibody and negative for brucellosis antibody) about 3-4 months old, 4 of which were challenged with the F4 generation of WM01 strain, and each sheep was inoculated with 4ml of WM01 culture (stock solution) in the trachea. ≥10 9.0 CCU / ml), the other 2 were used as controls, and the cultures without strains were injected into the trachea in the same way, and observed continuously for 35 days.
[0080] 2 days before the challenge and every morning and afternoon after the challenge, the rectal temperature of the experimental animals was measured, and the clinical symptoms were observed (at least 1 hour each time), including mental, appetite and respiratory system (such as cough, runny nose, etc.) symptoms. The weight of the sheep was weighed on day 0 of the challenge, and nasal swabs were collected ev...
Embodiment 3
[0084] The preparation of embodiment 3 ovine mycoplasma pneumoniae WM01 F6 strain inactivated vaccine
[0085] Take the culture of Mycoplasma ovis pneumoniae WM01 F6 strain and inoculate the modified Thiaucourt's broth medium at 5% v / v of the total medium, cultivate at 37°C for 4-5 days, and harvest when the pH drops to about 6.8-7.0. Carry out sterility and mycoplasma tests according to the appendix of "The Veterinary Pharmacopoeia of the People's Republic of China", the virus seeds should be pure, and the poison price should not be lower than 5×10 8 TCID 50 .
[0086] Mix 0.2mol / L BEA and 0.4mol / L NaOH in a volume ratio of 2:1 in a suitable container. Cyclize at 37±2°C for 1 hour to generate BEI, and adjust the pH to 7.2-7.6. Place the qualified culture medium in a container, add BEI inactivator at a final concentration of 3mM, inactivate for 36-40 hours, shake well, and then add sodium thiosulfate at a final concentration of 0.03M for neutralization. The inactivated ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com