Chemical pre-lithiation method of graphite electrodes for lithium-ion batteries
A lithium-ion battery and graphite electrode technology, applied in battery electrodes, electrochemical generators, graphite, etc., can solve the problems of affecting the first-week efficiency of the full battery, affecting the energy density of lithium-ion batteries, and low first-week efficiency, so as to promote Energy density and cycle stability, controllable lithiation depth, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
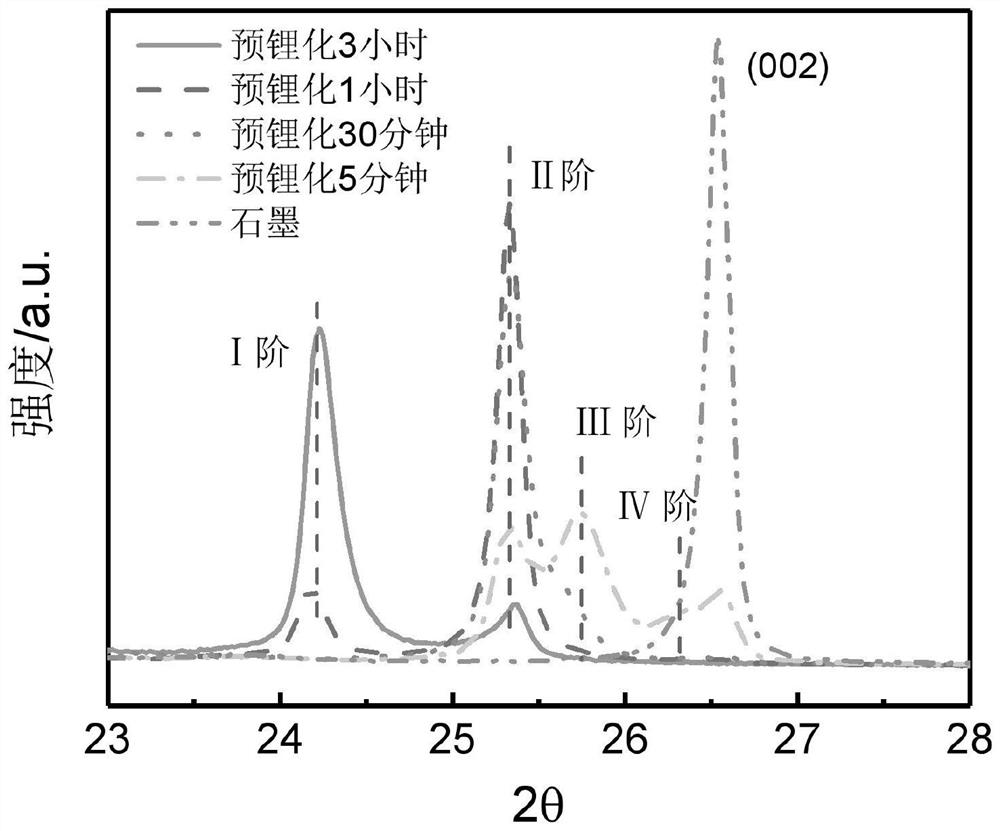
[0030] (1) The methyl propyl ether solution of 0.5mol / L naphthalene lithium of 1mL was reacted with natural graphite electrode (loading is about 2mg, containing graphite 93wt%) under the protection of inert atmosphere for different time (5min, 30min, 1h, 3h). After the reaction was complete, it was washed three times with methyl propyl ether and dried for later use.
[0031] (2) Carry out XRD detection to the above graphite electrodes with different lithiation depths, the scan rate is 4° / min, and the xrd spectrum is as follows figure 1 As shown, with the increase of time, the pre-lithiation depth of the graphite electrode gradually deepens, and it can even be lithiated to the first-order graphite (LiC 6 ).
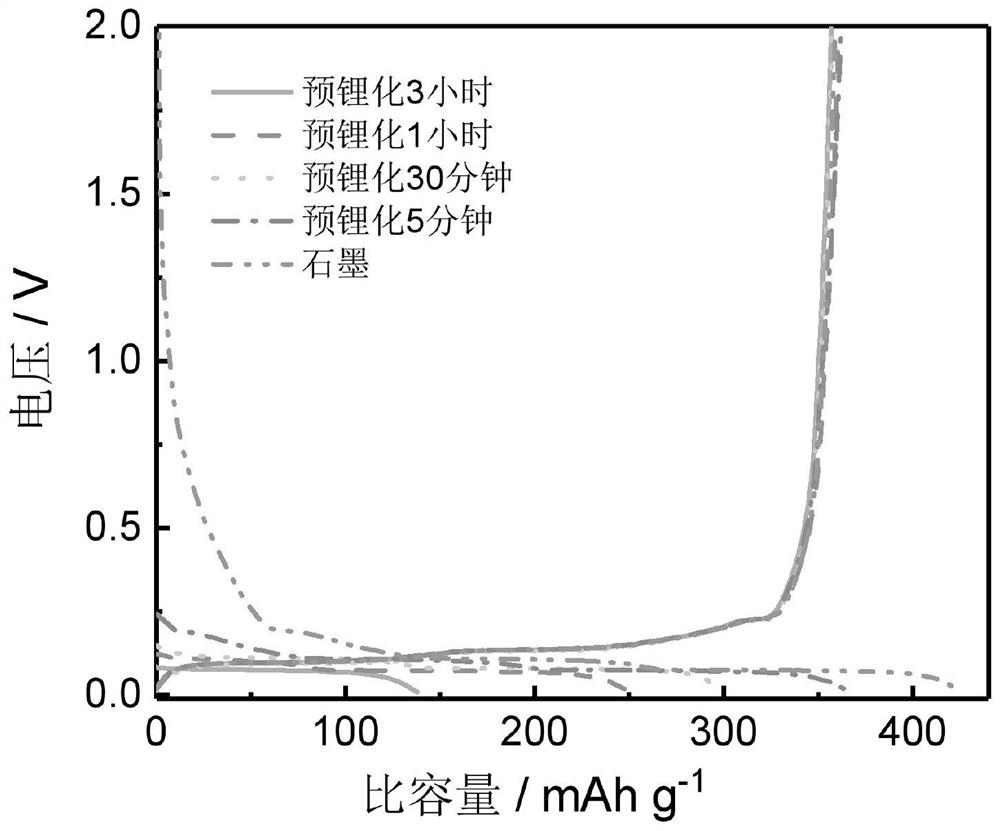
[0032] (3) Using natural graphite electrodes with different pre-lithiation depths as positive electrodes and lithium metal as negative electrodes, ternary electrolytes (1M LiPF 6 EC / DEC / DMC (v:v:v=1:1:1)) Assemble the half-cell, and conduct a charge-discharge test. Th...
Embodiment 2
[0034] (1) Take 3mL of 0.25mol / L tetramethylbiphenyllithium ethyl butyl ether solution and react with artificial graphite electrode (loading is about 2.5mg, containing graphite 95wt%) for 2 minutes under the protection of inert atmosphere. After the reaction was complete, it was washed three times with dimethyl carbonate and dried for later use.
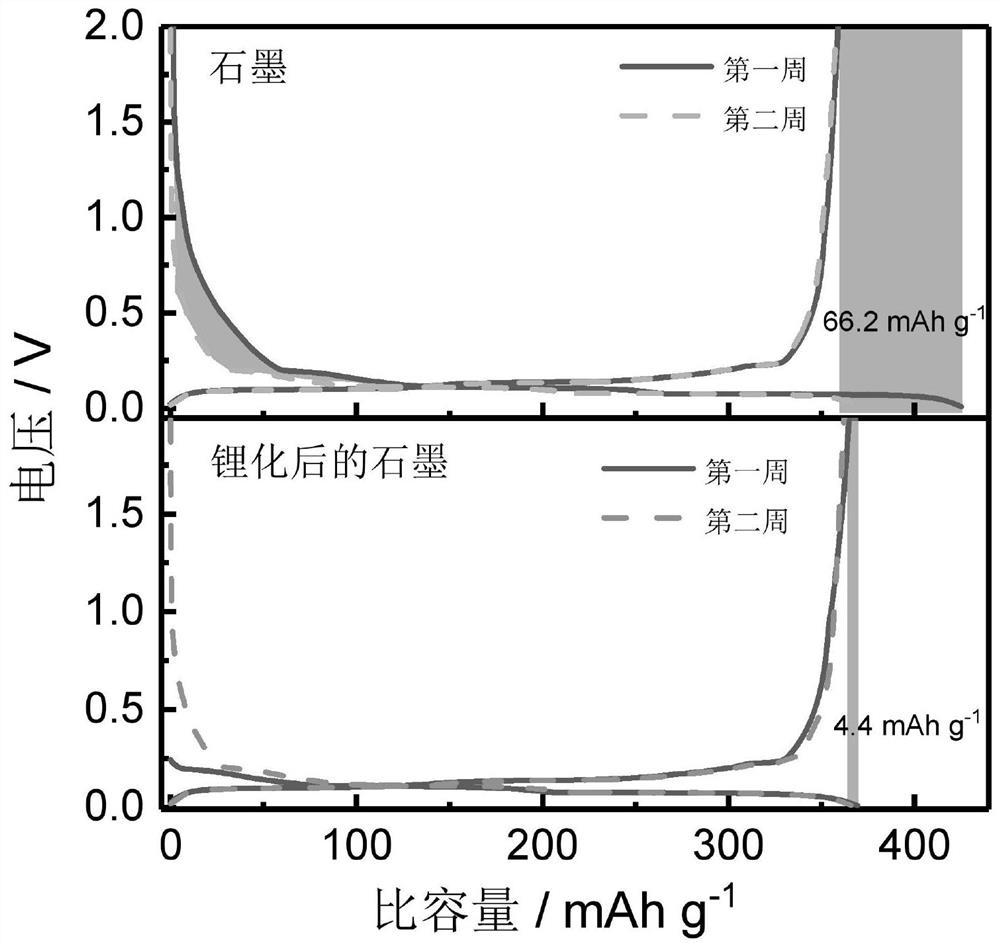
[0035] (2) The artificial graphite electrode before and after pre-lithiation is used as the positive electrode, and the lithium metal is used as the negative electrode, and the ternary electrolyte (1MLiPF 6 EC / DEC / DMC (v:v:v=1:1:1)) Assemble the half-cell, and conduct charge-discharge test. The charge and discharge curves and cycle diagrams of the previous two weeks are shown in 3 and Figure 4 As shown, after pre-lithiation, the open-circuit voltage of the half-cell decreased from 3.01V to 0.23V, and the efficiency increased from 84.45% to 98.91% in the first week. Moreover, the artificial graphite after pre-lithiation has faster ...
Embodiment 3
[0038] Take 3ml of 0.001mol / L anthracene-lithium propyl amyl ether solution and react with artificial graphite electrode (with a loading of about 2.5mg, containing 95wt% graphite) for 48h under the protection of an inert atmosphere. After the reaction was complete, it was washed three times with tetrahydrofuran and dried for later use.
[0039]The artificial graphite electrode before and after pre-lithiation was used as the positive electrode, and the lithium metal was used as the negative electrode, and the ternary electrolyte (1MLiPF 6 EC / DEC / DMC (v:v:v=1:1:1)) Assemble the half-cell, and conduct a charge-discharge test. After pre-lithiation, the open-circuit voltage of the half-cell decreased from 3.13V to 0.27V, and the efficiency increased from 85.03% to 99.31% in the first week.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com