Lithium ion battery safety additive and preparation method and application thereof
A lithium-ion battery and additive technology, which is applied in the direction of battery, secondary battery, secondary battery repair/maintenance, etc., can solve the problems of affecting the electrochemical performance of the battery and unsatisfactory flame retardant effect, so as to improve safety and good resistance. burn effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
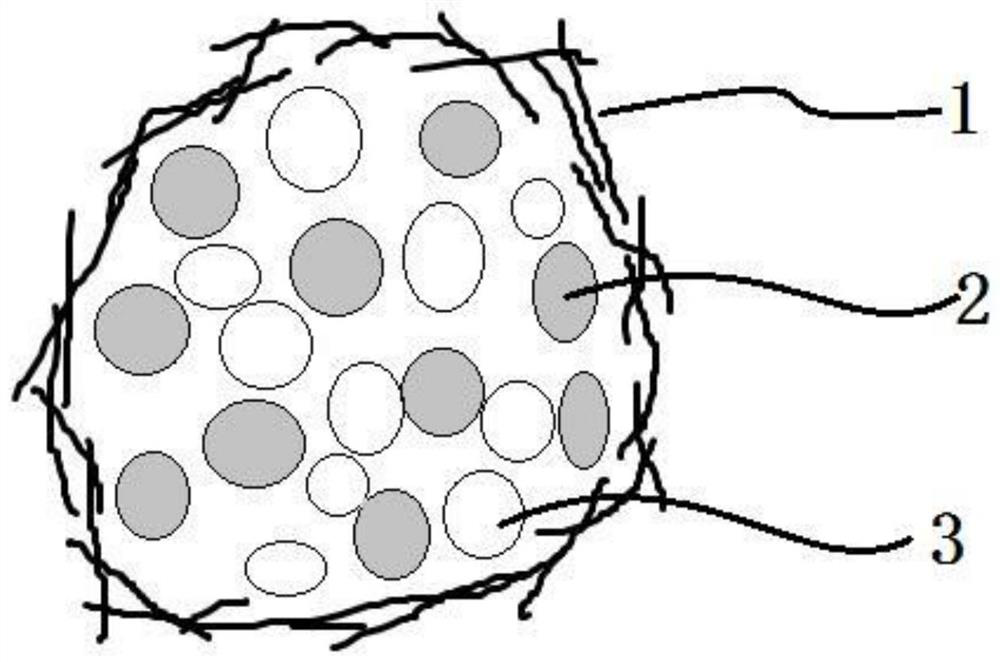
Image
Examples
Embodiment 1
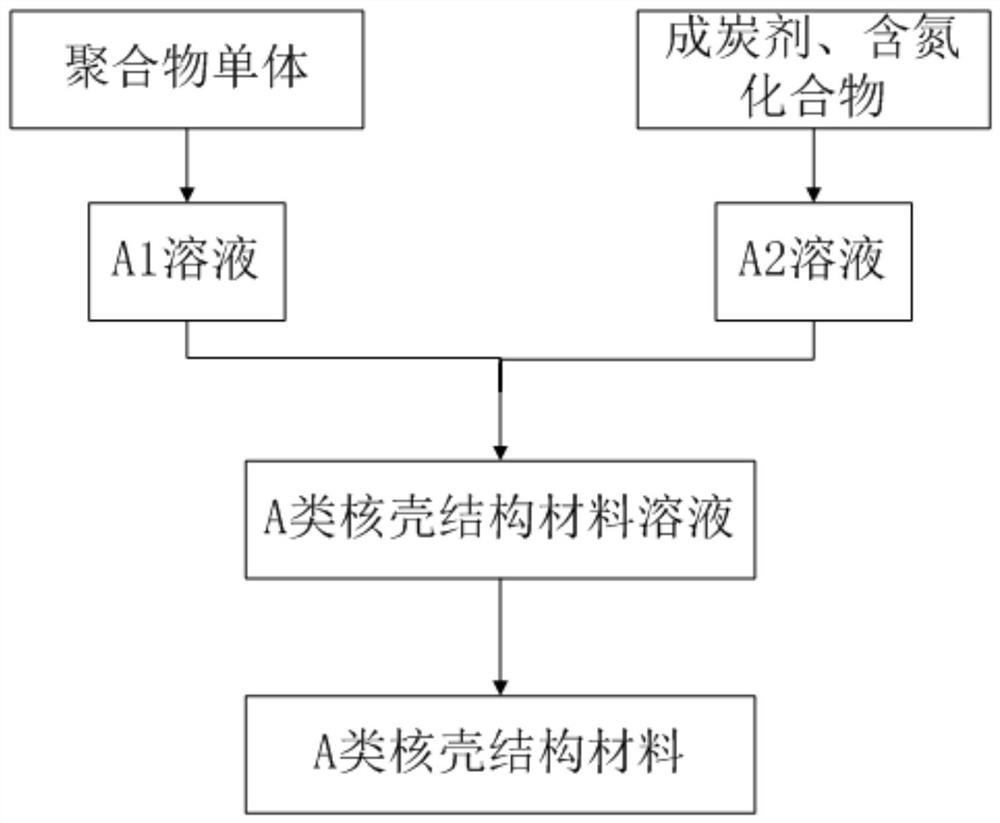
[0049] The specific synthesis steps of S11 and class A core-shell structure materials are as follows:
[0050] 1. Dissolve 10g caprolactone in 150mL toluene solution and disperse at room temperature for 60min to obtain solution A1;
[0051] 2. Disperse 6g of urea and 12g of phenolic resin in 150mL of toluene solution, and disperse at room temperature for 60min to obtain solution A2;
[0052] 3. Slowly add solution A1 to solution A2, stir evenly, add 0.1g benzoyl tert-butyl peroxide to polymerize caprolactone in situ, filter and use ethanol to remove impurities, and then place the solution in a fume hood After drying, the core-shell structure material of class A is obtained;
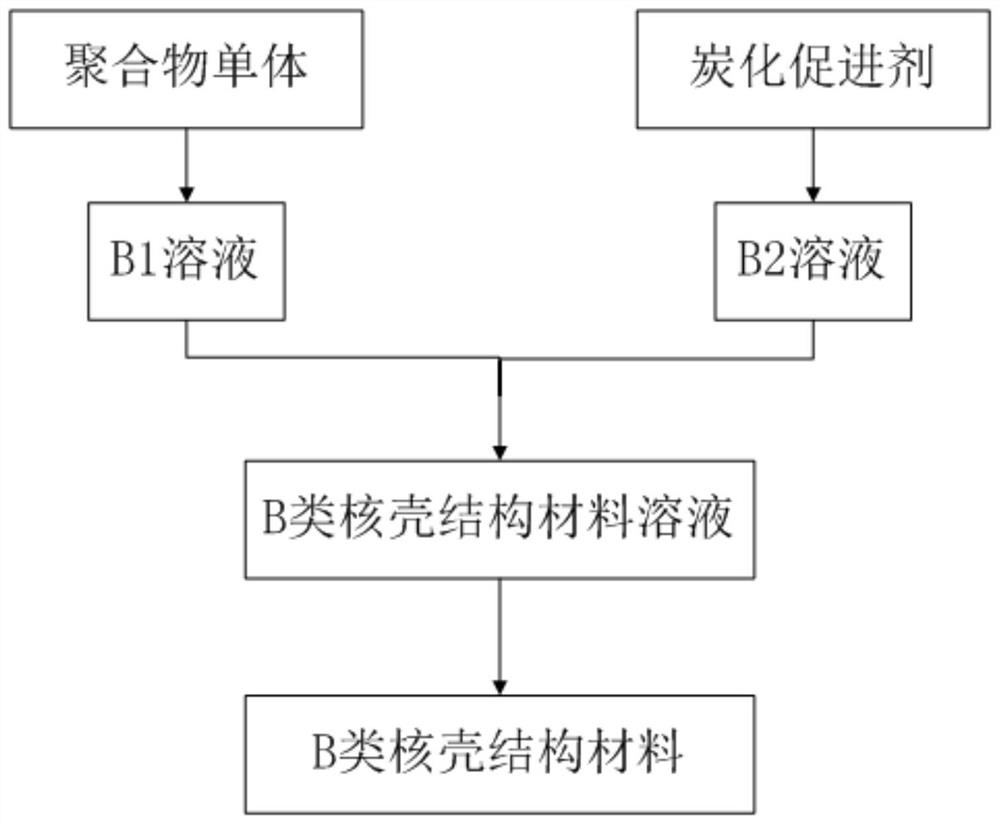
[0053] The specific synthesis steps of S12 and class B core-shell structure materials are as follows:
[0054] 1. Dissolve 6g caprolactone in 150mL toluene solution and disperse at room temperature for 60min to obtain solution B1;
[0055] 2. Dissolve 12g of phosphoric acid ester in 150mL of toluene so...
Embodiment 2
[0058] The specific synthesis steps of S21 and class A core-shell structure materials are as follows:
[0059] 1. Dissolve 5g caprolactone and 5g ethylene oxide in 150mL toluene solution, and disperse at room temperature for 60min to obtain solution A1;
[0060] 2. Disperse 6g of urea, 6g of ammonium polyphosphate, 3g of phenolic resin and 3g of pentaerythritol in 150mL of toluene solution, and disperse at room temperature for 60min to obtain solution A2;
[0061] 3. Slowly add solution A1 to solution A2, stir evenly, add 0.1g methyl ethyl ketone peroxide to polymerize caprolactone and ethylene oxide in situ, filter and use ethanol to remove impurities, and then place the solution in a fume hood After drying, the core-shell structure material of class A is obtained;
[0062] The specific synthesis steps of S22 and class B core-shell structure materials are as follows:
[0063] 1. Dissolve 3g caprolactone and 3g ethylene oxide in 150mL toluene solution, and disperse at room t...
Embodiment 3
[0067] The specific synthesis steps of S31 and class A core-shell structure materials are as follows:
[0068] 1. Dissolve 1g of ethylene oxide in 20mL of toluene solution and disperse at room temperature for 30min to obtain solution A1;
[0069] 2. Disperse 0.5g urea and 1g phenolic resin in 20mL toluene solution, and disperse at room temperature for 30min to obtain solution A2;
[0070] 3. Slowly add solution A1 to solution A2, stir evenly, add 0.01g ammonium persulfate to polymerize ethylene oxide in situ, filter and use ethanol to remove impurities, and then place the solution in a fume hood to dry to obtain Class A core-shell structure materials;
[0071] The specific synthesis steps of S32 and class B core-shell structure materials are as follows:
[0072] 1. Dissolve 1g of ethylene oxide in 20mL of toluene solution and disperse at room temperature for 30min to obtain solution B1;
[0073] 2. Dissolve 1g of phosphoric acid ester in 20mL of toluene solution and dispers...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com