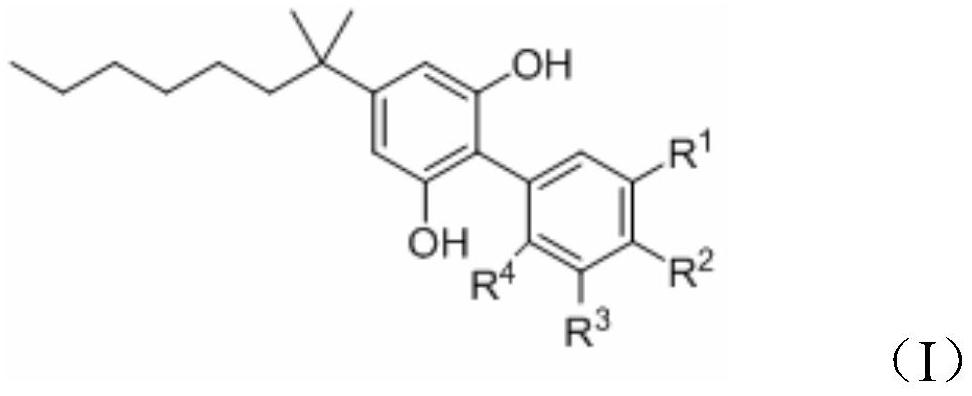
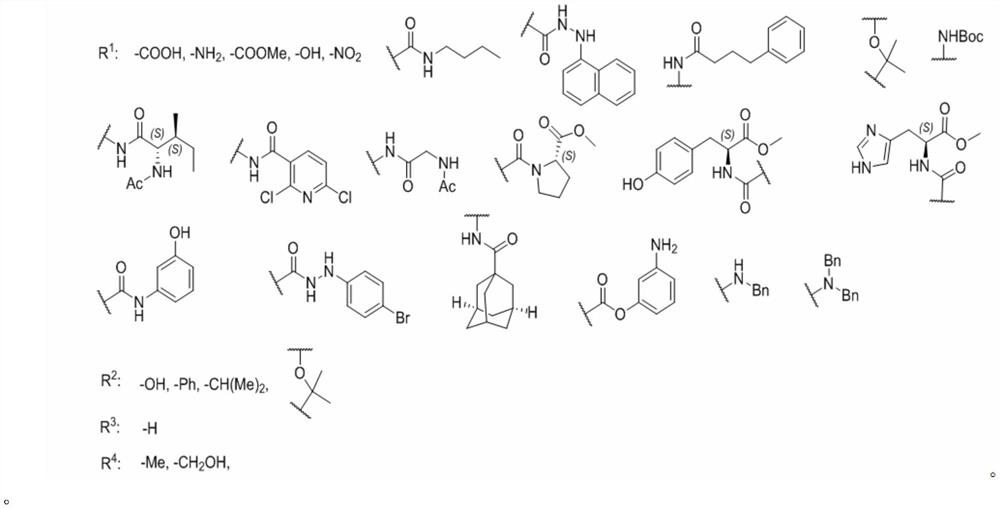
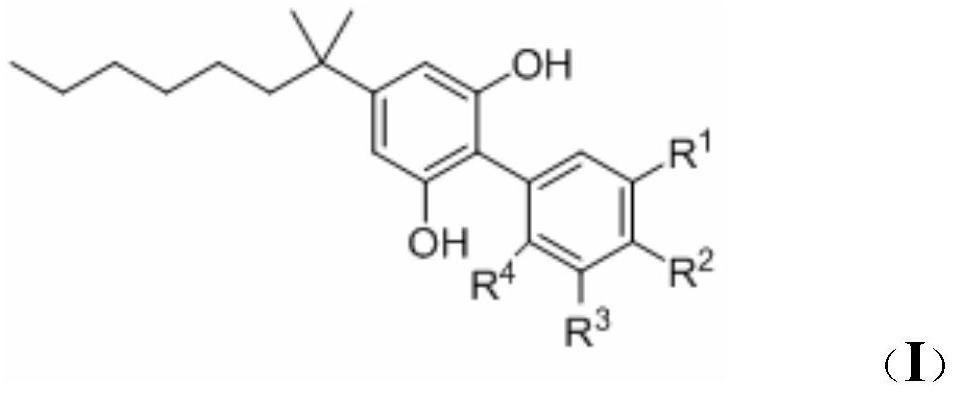
1, 1 '-biphenyl-2, 6-diphenol compound and application thereof
A technology of compound and diphenol, applied in the field of medicinal chemistry, can solve the problems of obvious toxic and side effects, multi-drug resistance, poor selectivity, etc., and achieve good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of compound 3: take 25ml reaction tube, add compound 2 (0.22mmol), compound 1 (0.18mmol), Cs 2 CO 3 (0.36mmol), Pd(PPh 3 ) 4 (0.02mmol), Ar gas was replaced with 1,4-dioxane (2ml) and placed in an oil bath at 80°C for 12h. TLC detected that the reaction was complete. Celite was filtered, the solvent was removed under reduced pressure, and compound 3 was obtained by separation and purification by column chromatography.
[0042] The preparation method of compounds I-1~I-6: Take a 25ml reaction tube, add compound 3 (0.18mmol), DCM 2mL, place in an ice ethanol bath (-15°C), slowly add 0.9ml BBr dropwise 3 (1.0M in DCM), naturally heated up for 12 hours, and TLC detected that the reaction was complete. The system was placed in an ice-water bath and quenched by adding 0.2ml of MeOH. Water and dichloromethane were then added, separated and extracted, dried over anhydrous sodium sulfate, concentrated under reduced pressure, separated and purified by ...
Embodiment 1
[0045] Compound I-1:
[0046] Preparation of compound 1 boronic acid: take a 250ml three-necked flask, add methyl 3-bromo-4-methylbenzoate (2.0 g, 8.77mmol), pinacol diboronic acid ester (4.46g, 17.54mmol), PdCl 2(dppf).DCM (143 mg, 0.175mmol), KOAc (3.44g, 35.08mmol) replace Ar gas first, add 1,4-dioxane (60 ml), and remove oxygen (diaphragm pumping system, replace Ar gas), placed in an oil bath at 80°C for 12 hours, and TLC detected that the reaction was complete. Filter with diatomaceous earth, remove the solvent under reduced pressure, add diethyl ether and water, separate and extract, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, separate and purify by column chromatography (Hexanes / EA=20:1 ) to obtain a relatively pure borate compound, and the excess boron reagent (Hexanes / EA) was removed by recrystallization to obtain a white solid compound 1 borate (1.81g, 75%). Add 20ml of methanol and...
Embodiment 2
[0050] Compound I-2
[0051] Using compound 2 and the corresponding boronic acid 1 as raw materials, I-2 (72mg, 63%) was obtained by referring to the method of compound I-1. 1 H NMR (400MHz, MeOD) δ: 6.84(d, J=1.7Hz, 1H), 6.81(d, J=8.1 Hz, 1H), 6.71(dd, J=8.1, 1.8Hz, 1H), 6.41(s ,2H),1.61–1.53(m,2H),1.32–1.19(m,6H),1.26(s,6H),1.17–1.05(m,2H),0.87(t,J=6.2Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com