Preparation method of pregabalin intermediate
A technology of pregabalin and intermediates, applied in the field of organic synthesis, can solve the problems of difficult industrialization, long reaction route and high cost, and achieve the effects of reducing production process, shortening steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A kind of preparation method of pregabalin intermediate, it comprises the steps:
[0055] Preparation of S1 intermediate 3-isobutylglutarimide
[0056]
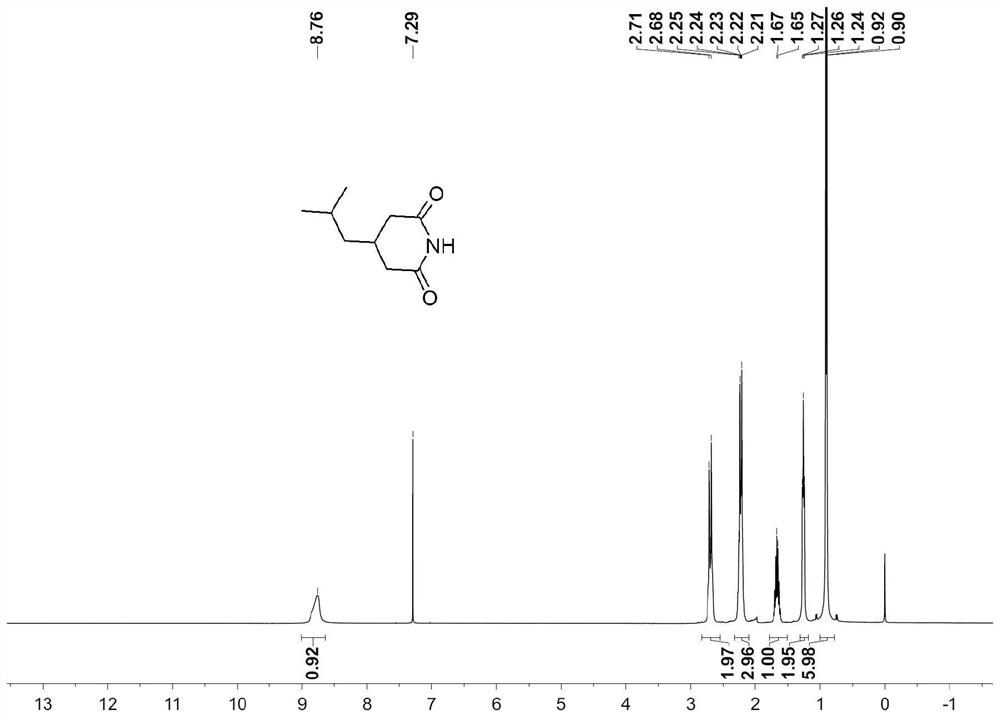
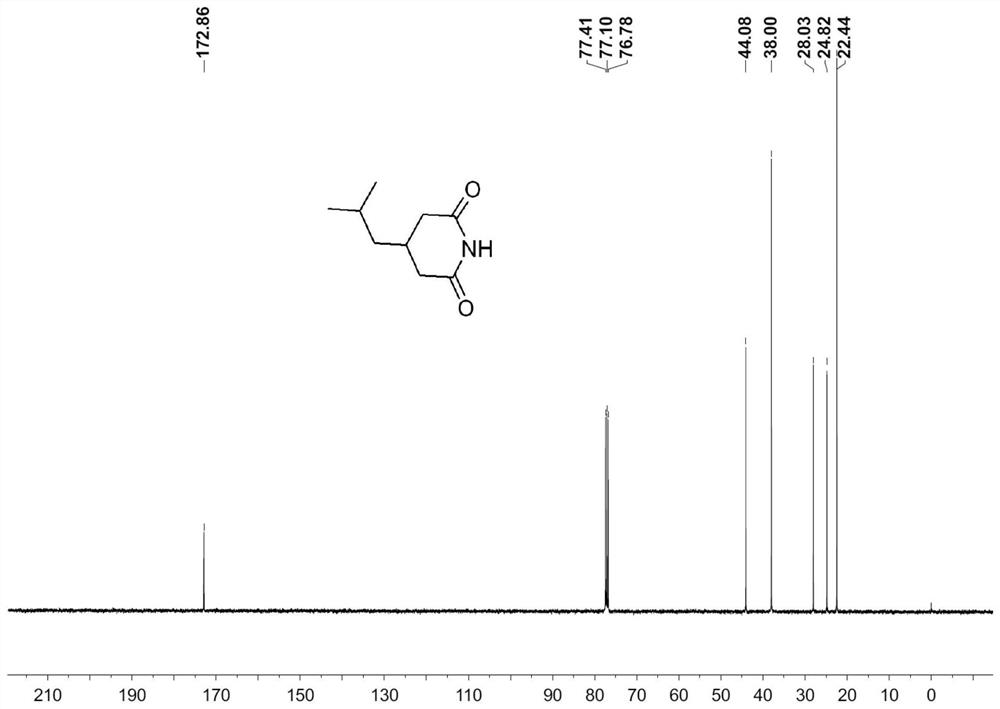
[0057] 249g of 70% cyanoacetic acid aqueous solution is cooled to below 10°C, and 300g of 30% lye is slowly added dropwise to control the internal temperature below 15°C. 86 g of isovaleraldehyde was slowly added dropwise. Insulated and stirred for 8h. Control the internal temperature not higher than 35°C, and slowly add 400g of concentrated sulfuric acid dropwise. Stir at 20-30°C for 0.5 hour, then at 60°C for 4 hours. The temperature was lowered to 20-30°C, and 300g of toluene was extracted twice. The organic phase was concentrated to obtain 158.0 g of crude 3-isobutylglutarimide, with a yield of 93.5% and a purity of 99.1%.
[0058] Such as Figure 1-2 Shown, is the intermediate 3-isobutylglutarimide 1 HNMR and 13 CNMR spectrum. It can be seen from the analysis that the detection result is consistent wit...
Embodiment 2
[0064] A kind of preparation method of pregabalin intermediate, it comprises the steps:
[0065] Preparation of S1 intermediate 3-isobutylglutarimide
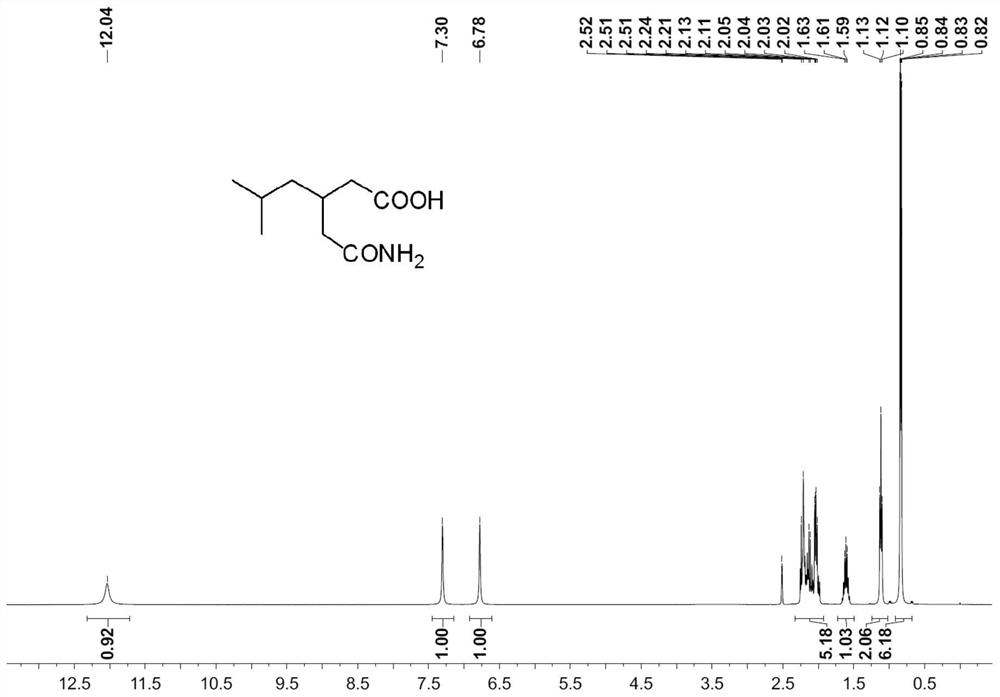
[0066] 249g of 70% cyanoacetic acid aqueous solution, cool down to below 10°C, slowly add 300g of 30% lye, control the internal temperature below 15°C; slowly add 86g of isovaleraldehyde, keep stirring for 20h, control the internal temperature not higher than At 35°C, 400g of concentrated sulfuric acid was slowly added dropwise. Stir at 20-30°C for 0.5 hour, then at 60°C for 4 hours. The temperature was lowered to 20-30° C., and 300 g of toluene was extracted twice; the organic phase was concentrated to obtain 154.1 g of crude 3-isobutylglutarimide, with a yield of 91.2% and a purity of 98.8%. Preparation of S2 3-(carbamoylmethyl)-5-methylhexanoic acid
[0067] Add 170g of crude 3-isobutylglutarimide and 60g of sodium hydroxide to 500g of water, slowly raise the temperature to 100°C, stir for 4-6h, cool to 10-20°C, slowly ad...
Embodiment 3
[0069] A kind of preparation method of pregabalin intermediate, it comprises the steps:
[0070] Preparation of S1 intermediate 3-isobutylglutarimide
[0071] 249g of 70% cyanoacetic acid aqueous solution is cooled to below 10°C, and 300g of 30% lye is slowly added dropwise to control the internal temperature below 15°C. 53 g of isovaleraldehyde was slowly added dropwise. Insulated and stirred for 8h. Control the internal temperature not higher than 35°C, and slowly add 400g of concentrated sulfuric acid dropwise. Stir at 20-30°C for 0.5 hour, then at 60°C for 4 hours. The temperature was lowered to 20-30°C, and 300g of toluene was extracted twice. The organic phase was concentrated to obtain 93.0 g of crude 3-isobutylglutarimide, with a yield of 89.3% and a purity of 95.5%.
[0072] Preparation of S2 3-(carbamoylmethyl)-5-methylhexanoic acid
[0073] Add 170g of crude 3-isobutylglutarimide and 36g of LiOH to 500g of water, slowly raise the temperature to 50-60°C, stir f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com