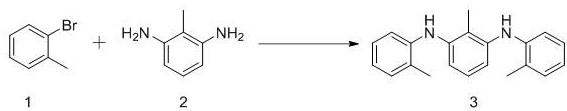Preparation method of elagolix intermediate
A technology of elagolix and intermediates, which is applied in the field of drug preparation, can solve problems such as the purification of aryl coupling intermediates, and achieve the effects of reducing the cost of process preparation, not easy to inactivate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a 5L three-necked reaction flask, add compound II, 500.0g, compound III, 238.2g, potassium carbonate 484g, and 2-methyl-N1, N3-di-o-tolylbenzene-1,3-diamine 35.3g , stirred and heated to 100°C and refluxed for 10 hours. Thin-layer chromatography showed that the conversion of the raw materials was complete, and the aqueous solution of acetic acid was added dropwise to the reaction solution. Compound I 478g, yield: 95.98%.
Embodiment 2
[0034] In a 2L three-necked reaction flask, sequentially add compound II, 100.00g, compound III, 47.64g, cesium carbonate 228.31g, 2-methyl-N1,N3-di-o-tolylbenzene-1,3-diamine 7.06g , stirred and heated to 60°C and refluxed for 6 hours. Thin-layer chromatography showed that the conversion of the raw materials was complete, and the aqueous solution of acetic acid was added dropwise to the reaction solution. Compound I 90.6g, yield: 91%.
[0035] Catalyst 2-methyl-N1, the synthetic route of N3-di-o-tolylbenzene-1,3-diamine is as follows:
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com