Purification method of baloxavir marboxil and derivatives thereof
A purification method and derivative technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of unfavorable preparation processing and ensure the uniformity of drug quality, easy condensation into small balls, and small particle size of the product, achieving significant cost-effectiveness , No electrostatic adsorption phenomenon, reduce the effect of solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0045] Embodiment 1-3 Purification method of baloxavir maple ester of the present invention
[0046] The purification parameters of Examples 1-3 are shown in Table 1.
[0047] The purification method of Example 1-3 comprises the following steps: add about 65g of baloxavir maplexate crude product to the 2L three-empty bottle, add the purification solvent, stir, heat up, and reflux to dissolve; naturally cool down to the required temperature, keep warm, Stand for crystallization for about 2 hours; filter with suction, rinse the filter cake with 100mL*2 times of purified water; dry in vacuum at 40°C for 15 hours to obtain purified products 1-3. The results are shown in Table 1.
[0048] Table 1
[0049]
[0050]
Embodiment 4
[0051] Embodiment 4 Purification method of baloxavir mapobate of the present invention
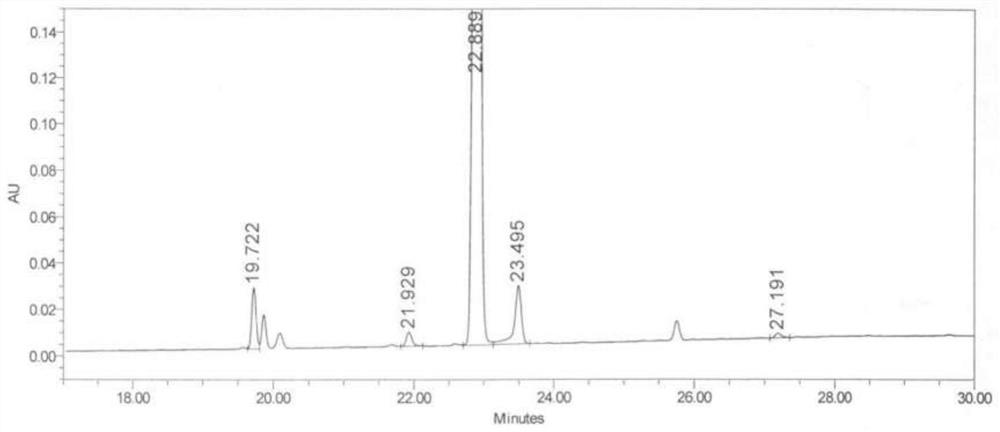
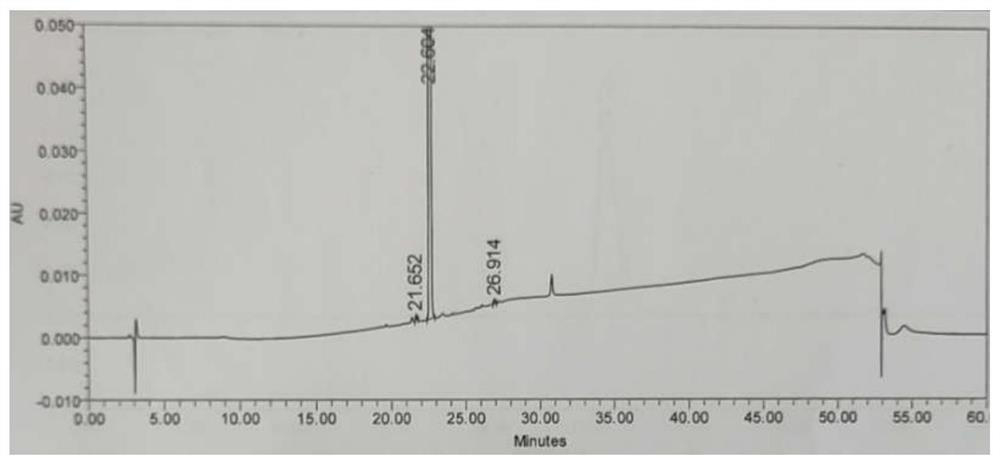
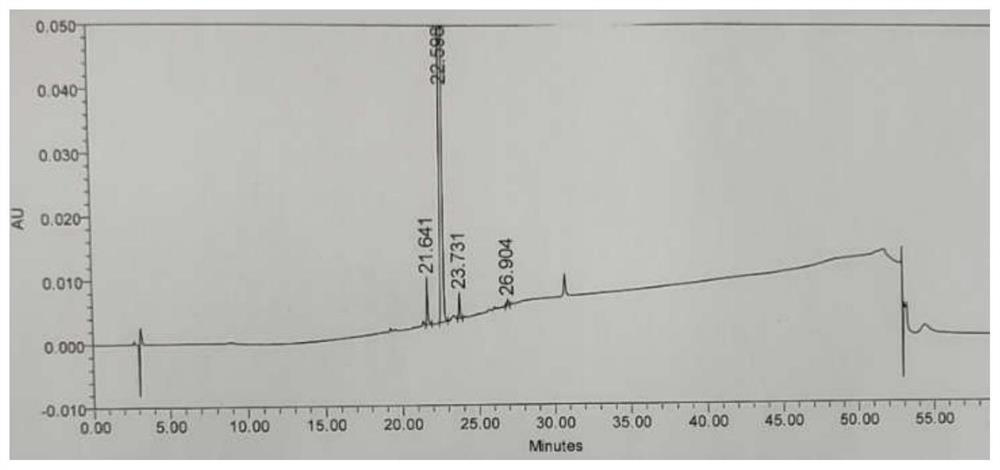
[0052] Add 65g of crude baloxavir maple ester to a 2L three-neck flask, add 1755mL of methanol, stir, raise the temperature to 65°C until it is completely dissolved, cool down to 0-5°C, keep warm and crystallize for 2 hours; filter, and use purified water for the filter cake 100mL*2 washes, and the filter cake was vacuum-dried at 40°C for 15 hours to obtain an off-white solid baloxavir maplelate. HPLC purity 99.9%.
Embodiment 5
[0056] The particle size distribution of the purified baloxavir maplelate prepared in Example 4 and Comparative Example 2 was detected by the following method.
[0057] Measuring instrument: Mastersizer 3000, main parameters: wet method, stirring speed 1000rpm, ultrasonic time 4min, shading degree 5%-20%, particle refractive index 1.52%. The results are shown in Table 2 and Figure 5-Figure 6 .
[0058] Table 2
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com