Method for preparing ferric chloride through rapid oxidation of ferrous chloride
A technology of ferrous chloride and ferrous chloride solution, which is applied in the chemical industry, can solve the problems of many impurities and low purity of ferric chloride, and achieve the effects of improving purity, reducing yield, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
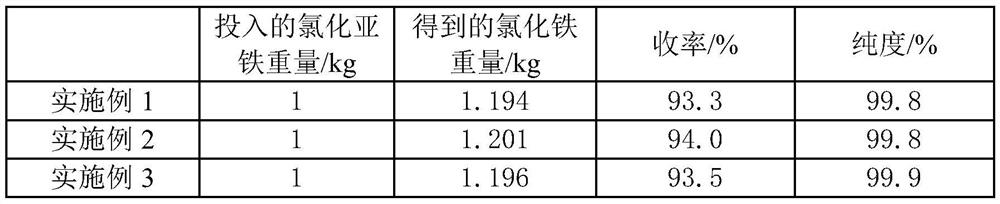
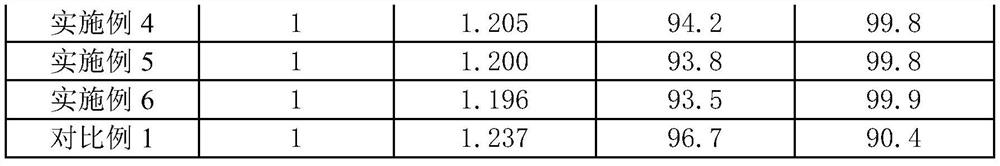
Embodiment 1
[0023] A kind of ferrous chloride fast oxidation prepares the method for ferric chloride:
[0024] Dissolve ferrous chloride derived from iron and steel hydrochloric acid pickling waste liquid with concentrated hydrochloric acid and filter to obtain a ferrous chloride solution with a pH of 1, and transfer the ferrous chloride solution to a sealed reactor. There is a circulation pump at the bottom, and the circulation pump is used to transport the ferrous chloride solution at the bottom of the reactor to the atomizer at the top of the reactor. Driven by the motor, the atomizer can atomize the ferrous chloride solution into small droplet, the reactor is also provided with a chlorine gas pipeline, the chlorine gas pipeline is used to transport the chlorine gas into the reactor to react with the atomized ferrous chloride solution, and the introduction direction of the chlorine gas is from bottom to top and The atomized ferrous chloride solution forms a convection current, and the ...
Embodiment 2
[0026] A kind of ferrous chloride fast oxidation prepares the method for ferric chloride:
[0027] Dissolve ferrous chloride derived from iron and steel hydrochloric acid pickling waste liquid with concentrated hydrochloric acid and filter to obtain a ferrous chloride solution with a pH of 1, and transfer the ferrous chloride solution to a sealed reactor. There is a circulation pump at the bottom, and the circulation pump is used to transport the ferrous chloride solution at the bottom of the reactor to the atomizer at the top of the reactor. Driven by the motor, the atomizer can atomize the ferrous chloride solution into small droplet, the reactor is also provided with a chlorine gas pipeline, the chlorine gas pipeline is used to transport the chlorine gas into the reactor to react with the atomized ferrous chloride solution, and the introduction direction of the chlorine gas is from bottom to top and The atomized ferrous chloride solution forms a convection current, and the ...
Embodiment 3
[0029] A kind of ferrous chloride fast oxidation prepares the method for ferric chloride:
[0030] Dissolve ferrous chloride derived from iron and steel hydrochloric acid pickling waste liquid with concentrated hydrochloric acid and filter to obtain a ferrous chloride solution with a pH of 1, and transfer the ferrous chloride solution to a sealed reactor. There is a circulation pump at the bottom, and the circulation pump is used to transport the ferrous chloride solution at the bottom of the reactor to the atomizer at the top of the reactor. Driven by the motor, the atomizer can atomize the ferrous chloride solution into small droplet, the reactor is also provided with a chlorine gas pipeline, the chlorine gas pipeline is used to transport the chlorine gas into the reactor to react with the atomized ferrous chloride solution, and the introduction direction of the chlorine gas is from bottom to top and The atomized ferrous chloride solution forms a convection current, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com