Construction and application of membraneless organelles in prokaryotes
An organelle and concatenated technology, which is applied to the construction and application of membraneless organelles in prokaryotes, can solve the problems of hindering the exchange of materials in compartments and increase the complexity of artificial synthesis, and achieve the effect of retaining biological activity and rapid material exchange.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Induces Escherichia coli to form membraneless compartments
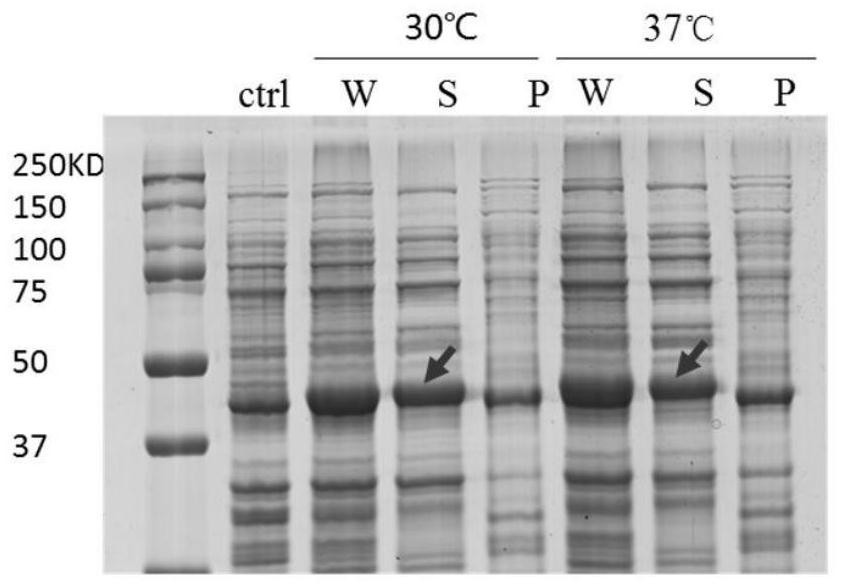
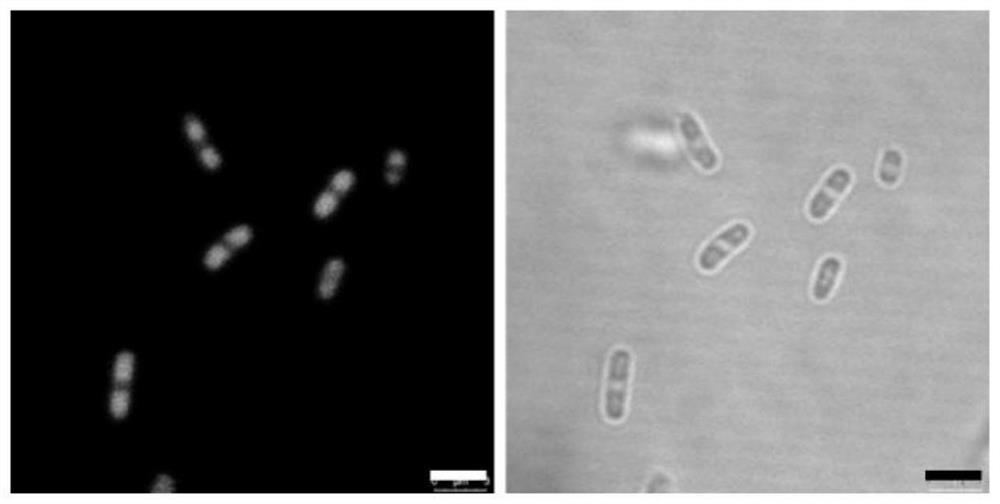
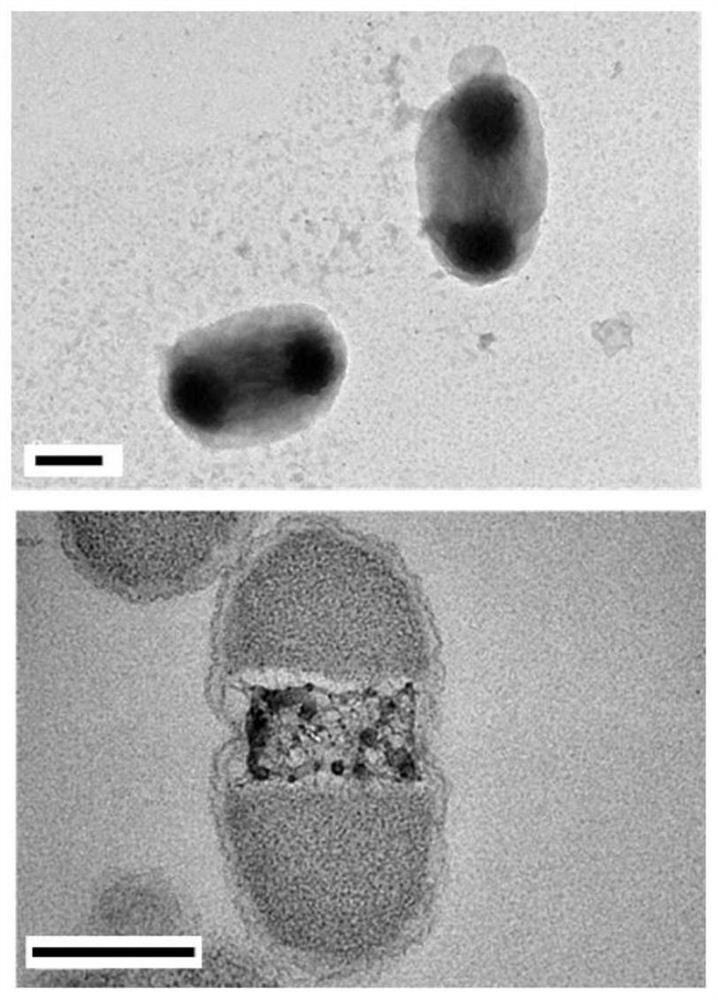
[0032] The specific steps of this example include: constructing a protein expression vector pET28a4-MaspI16 composed of 16 repeat peptide monomers of spider dragline protein MaSp1 (amino acid composition is GRGGLGGQGAGAAAAAGGAGQGGYGGLGSQG), and transforming it into the expression host cell Escherichia coli BL21 In (DE3), the recombinant expression strain was cultured overnight at 37°C / 220rpm in 4 mL LB medium containing kanamycin (0.05mg / mL), and transferred to 20mL medium containing kanamycin at an inoculum size of 1%. In LB medium, culture at 37°C / 220rpm until OD600 is about 0.6, add 200μM IPTG, continue to culture for 6h, take samples, and verify the solubility of the target protein by SDS-PAGE, such as figure 1 ; Escherichia coli intracellular compartments were visualized under a microscope by Tht staining, as figure 2 ; Observation of E. coli intracellular compartments by transmission electron microscop...
Embodiment 2
[0039] Construction of fluorescently active membrane-free compartments
[0040] The specific steps of this example include: construction of 16 recombinant spidroin protein Masp1 repeat peptide concatenations and GFPmut protein fusion protein expression vector pET28a4-MaspI16-gfp; the GFPmut peptide of the fusion protein is connected as shown in Seq ID No.4 The peptide (the amino acid composition is GGSGGSGGSGGS) is connected to the C-terminus of the peptide concatenation.
[0041] The above expression vector was transformed into the expression host cell Escherichia coli BL21 (DE3), and the recombinant expression strain was cultured overnight at 37° C. / 220 rpm in 4 mL LB medium containing kanamycin (0.05 mg / mL) to Transfer 1% of the inoculum into 20mL LB medium containing kanamycin, cultivate at 37℃ / 220rpm until the OD600 is about 0.6, add 200μM IPTG, continue to cultivate for 6h, take samples, and observe the intracellular region of Escherichia coli under a fluorescent microsc...
Embodiment 3
[0044] Colocalization of two fluorescent proteins in the membrane-free compartment
[0045]The specific steps of this example include: constructing 16 recombinant spidroin protein Masp1 repeat peptide concatenations and GFPmut protein fusion protein expression vector PET28a4-MaspI16-gfp; constructing 16 recombinant spidroin protein Masp1 repeat peptide concatenations fused with mcherry protein Protein expression vector PACYC-MaspI16-rfp; construction of 16 recombinant spidroin protein Masp2 repeat peptide concatenations and mcherry protein fusion protein expression vector PACYC-MaspII16-rfp; construction of 32 arthropod elastin-like conserved peptide concatenations and mcherry protein Fusion protein expression vector PACYC-R32-rfp
[0046] Transform any expression vector of pET28a4-MaspI16-gfp and pACYC-MaspI16-rfp / pACYC-MaspII16-rfp / pACYC-R32-rfp into the expression host cell Escherichia coliBL21 (DE3), and the recombinant expression strain Cultivate overnight at 37°C / 220rpm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com