Tumor cell vaccine targeting cafs, preparation method and application thereof
A tumor cell vaccine and tumor cell technology, applied in the direction of tumor/cancer cells, botany equipment and methods, biochemical equipment and methods, etc., can solve the problem that tumor vaccines are not suitable for mass production and transformation applications, and the antigen expressed by tumor cells is not stable enough , unfavorable tumor immune activation and other issues, to achieve the effect of enhancing radiosensitivity, improving fibrosis, and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A method for preparing a tumor cell vaccine targeting CAFS, comprising the following steps:
[0055] The NCBI gene number of FAP (fibroblast activation protein) is NM_007986.3, and the length of its coding region is 2285bp; PCR amplification is carried out with FAP cDNA as a template: the primer sequences used are shown in Table 1 below:
[0056] Table 1 Primer sequences used in PCR:
[0057]
[0058] Mix the following solutions in a PCR tube:
[0059]
[0060] The negative control is corresponding to no template; PCR reaction conditions: 98°C, 10sec, 55°C, 5sec, 72°C, 30sec, a total of 35 cycles, then 72°C for 2min, 16°C for 2min;
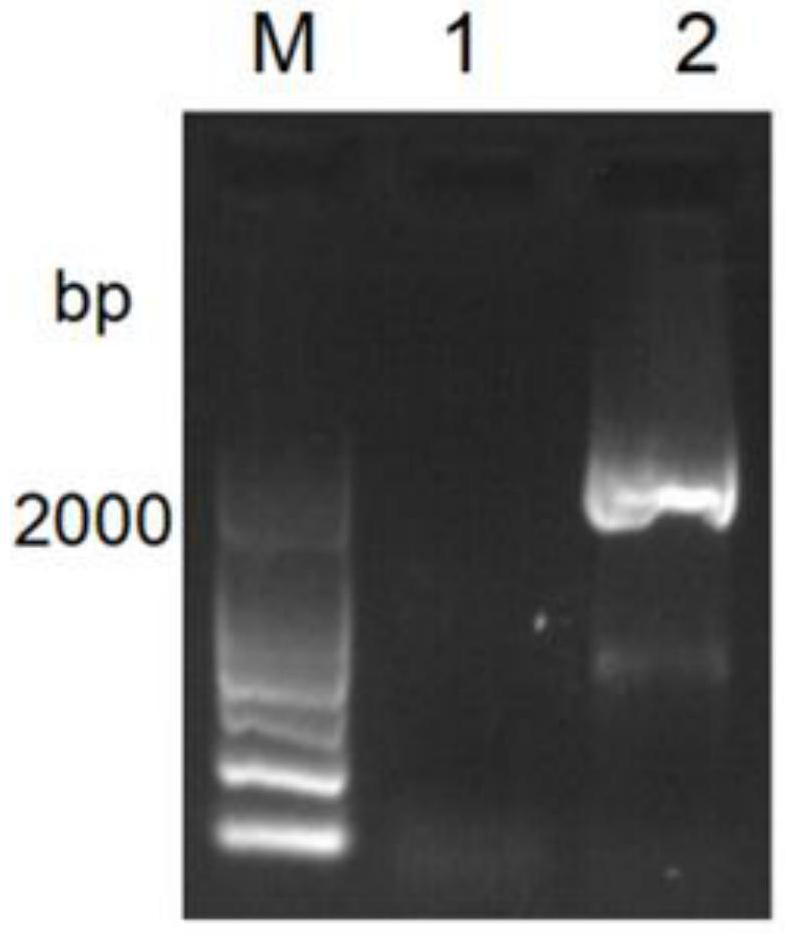
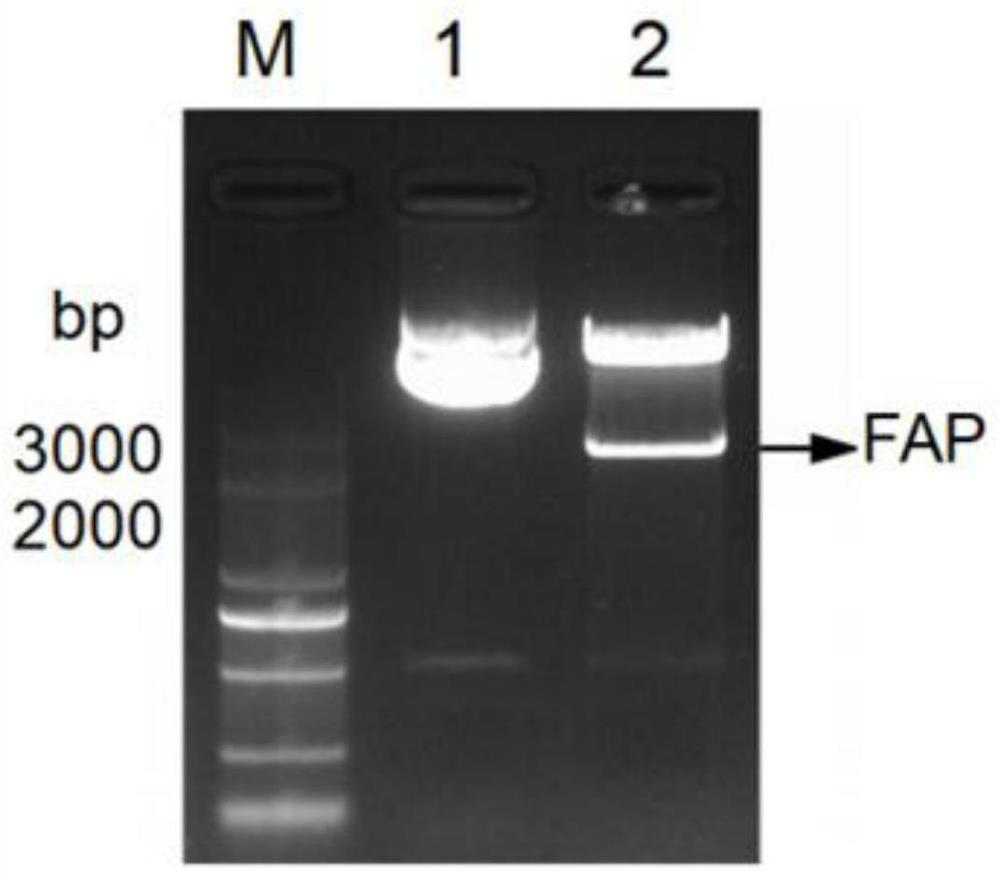
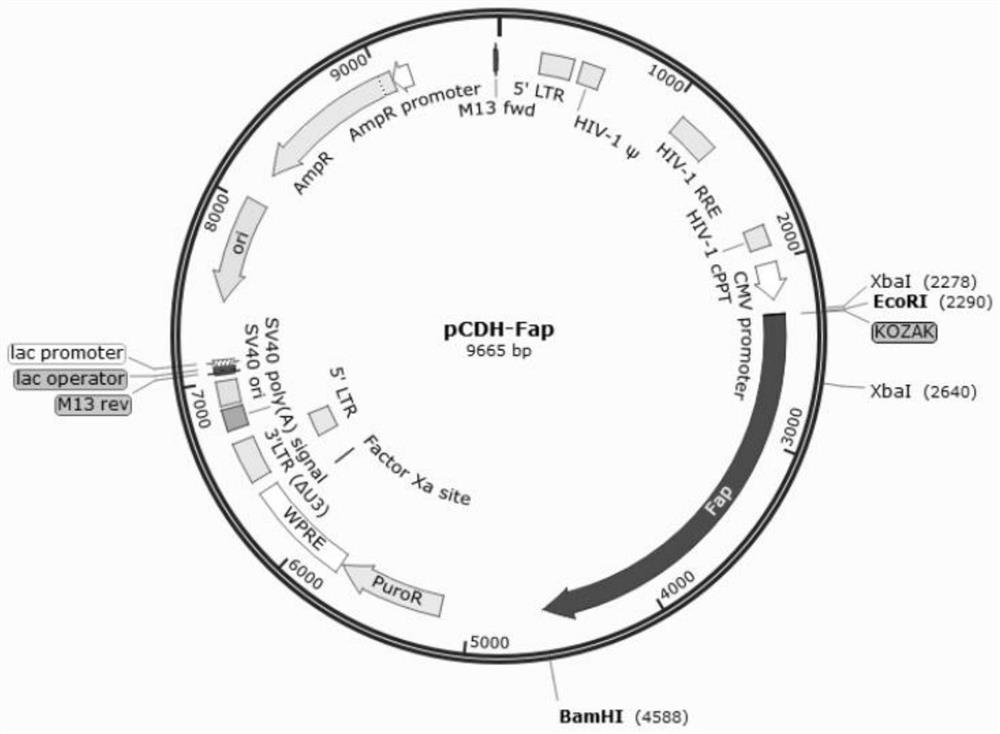
[0061] Through the above PCR reaction, amplified fragments with consistent band sizes were successfully amplified, such as figure 1 As shown in band 2 in the middle, this PCR amplified fragment was seamlessly cloned and connected to the lentiviral vector pCDH digested with EcoRI and BamHI to construct the lentiviral vector pCDH-FA c...
Embodiment 2
[0065] The inventor's previous research found that a tumor cell vaccine expressing human FAPα can inhibit tumor growth and improve tumor interstitial fibrosis, but the efficacy of the vaccine alone is limited. Studies have shown that SABR combined with tumor-specific vaccines will produce an in situ vaccine effect and further improve the body's anti-tumor immune response. The therapeutic effect is affected by factors such as radiotherapy regimens and the timing of combined treatment.
[0066] This example established a mouse LLC lung cancer model, compared the anti-tumor effects of different SABR regimens (single dose of 16.4Gy×1 time or single dose of 8Gy×3 times) and different treatment sequences, and observed tumor-bearing mice Tolerance to treatment.
[0067] Experimental animal grouping: 6 days after tumor inoculation, 48 mice were randomly divided into 8 groups, 6 in each group, as follows: Figure 6 as shown,
[0068] The specific method is as follows: female C57 / bl m...
Embodiment 3
[0071] In this example, mouse 4T1 breast cancer and CT26 colon cancer subcutaneous tumor models were established, and it was further clarified that the therapeutic effect of tumor cell vaccine expressing human FAP combined with SABR was superior to radiotherapy or vaccine immunotherapy alone.
[0072] Female Balb / c mice aged 6-8 weeks were selected and subcutaneously inoculated with 4×10 5 / 100ml 4T1 breast cancer or CT26 colon cancer cells. On the 8th day, 30 mice were randomly divided into 5 groups, 6 in each group, specifically:
[0073] Untreated group: control group
[0074] SABR group: start ablative radiotherapy on the 10th day 8Gy×3 times
[0075] Vaccine group: Immunotherapy with subcutaneous injection of tumor cell vaccine expressing human FAP on days 7, 14, and 21
[0076] SABR+pCDH group: On the 10th day, ablation radiotherapy 8Gy×3 times was started, and on the 7th, 14th, and 21st days, the tumor cells infected with the inactivated pCDH empty vector were used f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com