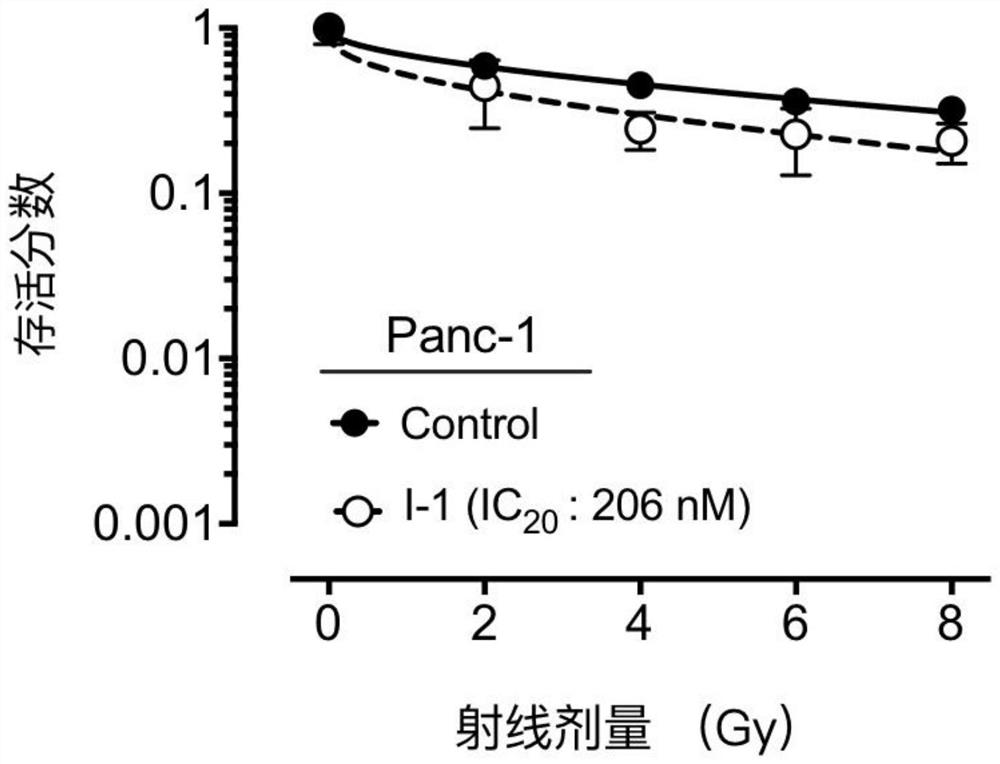
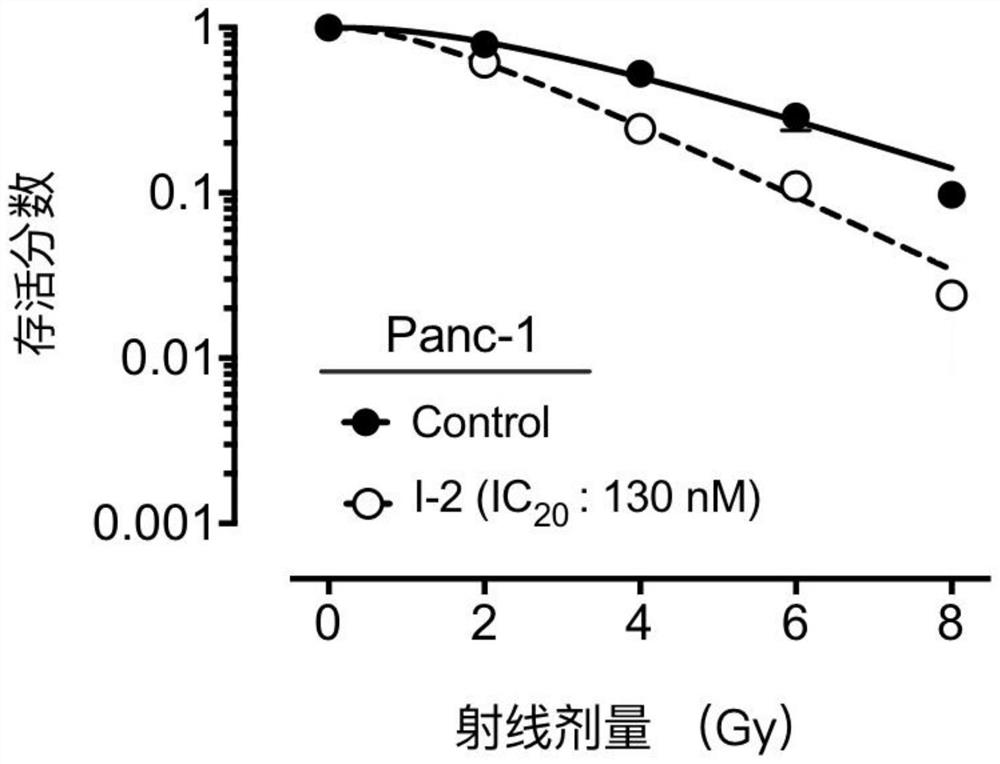
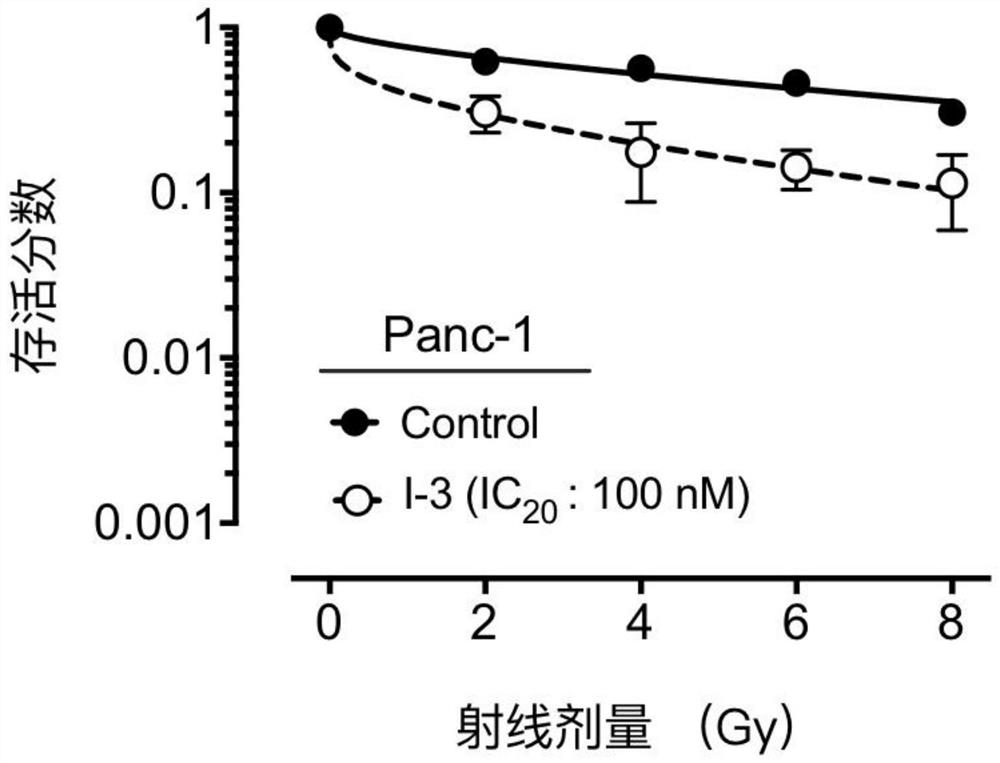
A compound containing a seven-membered lactam ring and its application
A technology of lactam rings and compounds, applied in the field of medicine, can solve the problems of high local recurrence and radiation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of 3-chloro-1-phenylallyl-6,7-dihydro-1H-azepine-2(5H)-one, the synthetic route is as follows:
[0049]
[0050] Weigh 62 mg of cinnamic acid 1 and dissolve in anhydrous dichloromethane (2 mL), add oxalyl chloride (0.18 mL) and a catalytic amount of DMF. After stirring at room temperature for 2 hours, the solvent was evaporated under reduced pressure to obtain compound 2, which was directly used in the next step.
[0051] The compound 3-chloro-6,7-dihydro-1H-azepine-2(5H)-one (3,68mg) was dissolved in anhydrous tetrahydrofuran (5mL), cooled to -78°C and slowly added n-butyl Lithium tetrahydrofuran solution (2.5M, 0.2mL), continued the reaction for 15 minutes, and then added dropwise the anhydrous tetrahydrofuran solution of compound 2 obtained in one step. Slowly rise to room temperature and react for 30 minutes, quenched, extracted with dichloromethane, dried, and evaporated under reduced pressure to obtain a crude product, which was separated by chro...
Embodiment 2
[0053] (E)-N-(4-(3-(6-Chloro-7-oxo-2,3,4,7-tetrahydro-1H-azaheptacycline-1-yl)-3-oxopropene- 1-base) the preparation of phenyl) acrylamide, synthetic route is as follows:
[0054]
[0055] Weigh 62 mg of compound 4 and dissolve in anhydrous dichloromethane (2 mL), add oxalyl chloride (0.18 mL) and a catalytic amount of DMF. After stirring at room temperature for 2 hours, the solvent was evaporated under reduced pressure to obtain compound 5, which was directly used in the next step.
[0056] The compound 3-chloro-6,7-dihydro-1H-azepine-2(5H)-one (3,68mg) was dissolved in anhydrous tetrahydrofuran (5mL), cooled to -78°C and slowly added n-butyl Lithium tetrahydrofuran solution (2.5M, 0.2mL), continued to react for 15 minutes, and then added dropwise the anhydrous tetrahydrofuran solution of compound 5 obtained in one step. Slowly rise to room temperature and react for 30 minutes, quenched, extracted with dichloromethane, dried, evaporated the solvent under reduced pressure...
Embodiment 3
[0060] (E)-2-Chloro-N-(4-(3-(6-Chloro-7-oxo-2,3,4,7-tetrahydro-1H-azaheptane-1-yl)-3- Preparation of oxypropen-1-yl) phenyl) acetamide:
[0061]
[0062] Dissolve Intermediate 7 (57 mg) in dichloromethane (10 mL), add 18 μL of triethylamine, react at 0°C for 10 minutes, slowly add 35 μL of chloroacetyl chloride, react at 0°C for 4 hours, extract with dichloromethane, wash with water , separated by chromatographic column to obtain compound I-3, light yellow solid, yield, 68%. 1 H NMR (300MHz, CDCl 3 )δ: 1.98-2.04 (m, 2H, J=6.3Hz, CH 2 ), 2.34-2.41 (q, 2H, J=7.0Hz, CH 2 ),3.97-4.02(t,2H,J=6.4Hz,NCH 2 ), 5.78-5.82 (d, 1H, J = 10.5Hz, = CH), 6.21-6.30 (dd, 1H, J 1 =10.0Hz,J 2 =10.1Hz, =CH), 6.43-6.49(d,1H,J=17.0Hz,=CH),6.76-6.81(t,1H,J=7.8Hz,=CH),7.39-7.44(d,2H, J=15.5Hz, Ar-H), 7.53-7.56(d, 2H, J=8.6Hz, Ar-H), 7.60-7.64(d, 2H, J=8.7Hz, Ar-H), 7.74-7.79( d,1H,J=15.5Hz,=CH); 13 C NMR (75MHz, CDCl 3 )δ: 23.82, 26.04, 41.45, 118.99, 119.97, 128.61, 129.60, 130.99, 131.05...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com