Non-coplanar benzimidazole diamine and preparation method thereof
A benzimidazole diamine, non-coplanar technology, which is applied in the field of non-coplanar benzimidazole diamine and its preparation, can solve the problems of few types of non-coplanar benzimidazole diamine, unsatisfactory, single structure and the like, Achieve the effect of improving optical properties and solubility, reducing conjugation effect and overcoming single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The present invention provides the preparation method of non-coplanar benzimidazole diamine described in the above scheme, comprising the following steps:
[0047] (1) performing a condensation reaction between a compound having a structure shown in formula a and a compound having a structure shown in formula b, and subjecting the resulting condensation product to a ring-closing reaction to obtain a compound having a structure shown in formula c;
[0048]
[0049] The types of X and Y in formula a to formula c are consistent with those in formula I;
[0050] (2) Under the condition of hydrogen, the compound having the structure shown in formula c is subjected to catalytic hydrogenation reaction to obtain the non-coplanar benzimidazole diamine having the structure shown in formula I.
[0051] The synthetic route of the present invention's synthetic non-coplanar benzimidazole diamine is shown in formula A, and is specifically described below in conjunction with formula...
Embodiment 1
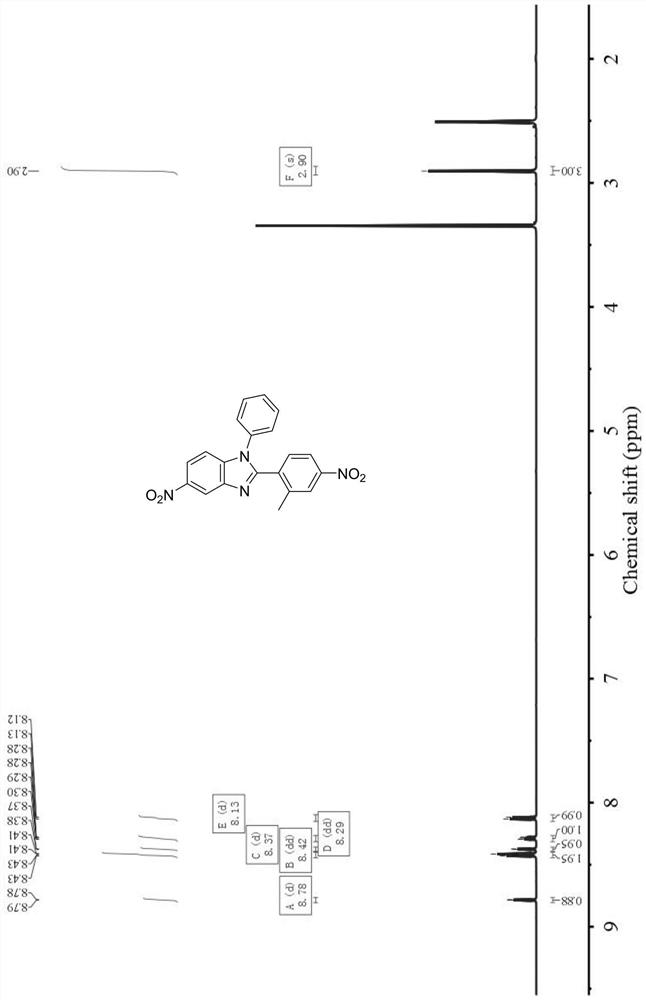
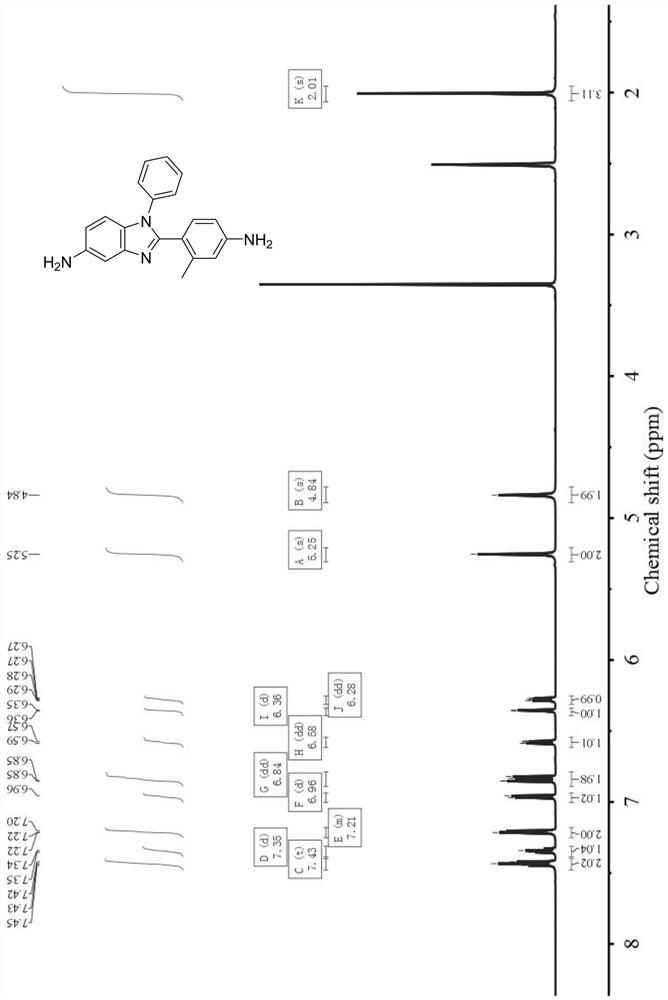
[0069] In this example, the specific structure of the non-coplanar benzimidazole diamine is as follows, and the Chinese name is 5-amino-2-(2-methyl-4-aminobenzene)-1-phenylbenzimidazole:
[0070]
[0071] The preparation method of above-mentioned 5-amino-2-(2-methyl-4-aminobenzene)-1-phenylbenzimidazole is as follows:
[0072] (1) Add 50.0g of N-phenyl-4-nitrobenzene-1,2-diamine, 33.1g of triethylamine and 500.0g of dichloromethane into the reaction flask, cool down to 5±1°C, and drop 52.1 g 3-methyl-4-nitrobenzoyl chloride, the dropwise addition was completed in 2-3 hours, reacted at room temperature for 16 hours, and TLC confirmed that the reaction was complete. Add 1000.0g of water to the reaction system, filter under reduced pressure to obtain 85.6g of crude product, add 800.0g of butyrolactone and 45.0g of p-toluenesulfonic acid after drying, react at 200°C for 4h, TLC confirms that the reaction is complete, cool down to 0~ 5°C, add 1400.0g water to the reaction syste...
Embodiment 2
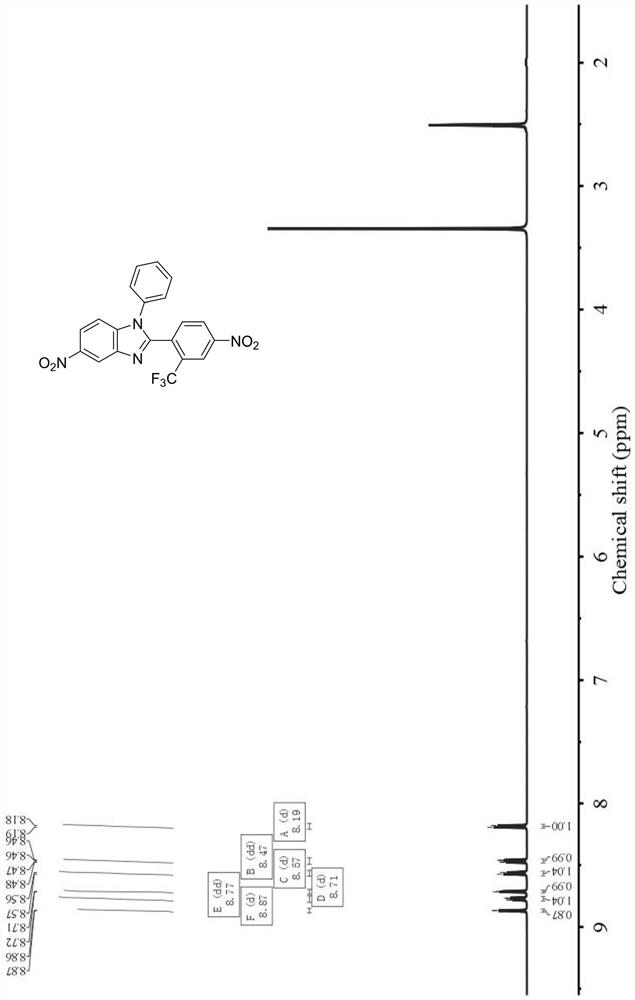
[0075] In this example, the specific structure of the non-coplanar benzimidazole diamine is as follows, and the Chinese name is 5-amino-2-(2-trifluoromethyl-4-aminobenzene)-1-phenylbenzimidazole:
[0076]
[0077] The preparation method of above-mentioned 5-amino-2-(2-trifluoromethyl-4-aminobenzene)-1-phenylbenzimidazole is as follows:
[0078] (1) Add 50.0g N-phenyl-4-nitrobenzene-1,2-diamine, 25.9g pyridine and 500.0g dichloromethane to the reaction flask, cool down to 5±1°C, add dropwise 66.3g 3- Trifluoromethyl-4-nitrobenzoyl chloride was added dropwise in 2-3 hours, and reacted at room temperature for 16 hours, and TLC confirmed that the reaction was complete. Add 1000.0g water to the reaction system, filter under reduced pressure to obtain 97.0g crude product, add 1000.0g sulfolane and 21.8mL concentrated hydrochloric acid after drying, react at 200°C for 4h, TLC confirms that the reaction is complete, cool down to 0-5°C, and pour Add 1400.0 g of water to the reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com