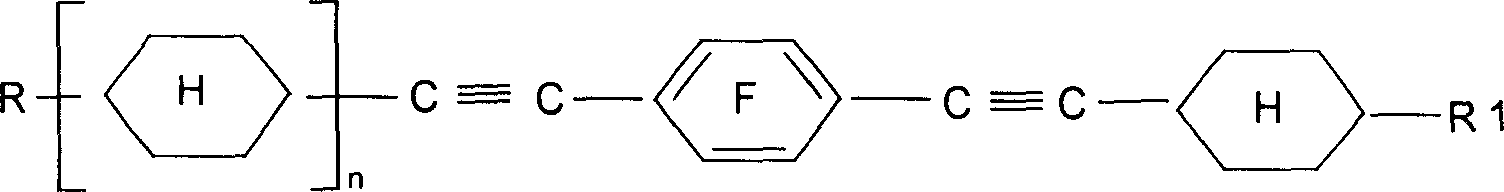
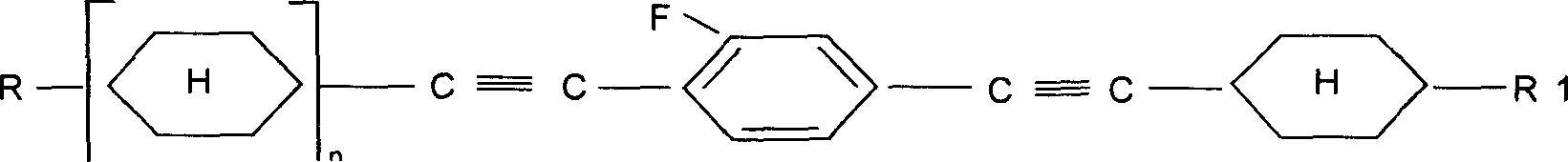
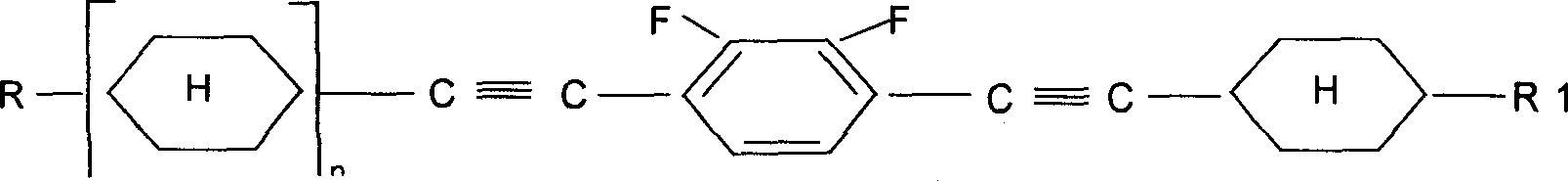
Alkyl cyclohexyl alkynes liquid crystal compound and its preparation method
A technology of liquid crystal compounds and alkyl rings, applied in chemical instruments and methods, liquid crystal materials, etc., can solve the problems of unsatisfactory fat solubility, unfavorable high-speed response of liquid crystal display, high viscosity of the system, etc., and achieve reduced rigidity and high resistivity , good fat-soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Preparation of liquid crystal monomer 1-[(trans)-4-ethylcyclohexylethynyl]-2-fluoro-4-[(trans)-4'-n-propylcyclohexylethynyl]benzene.
[0018] 1. From (anti)-4-ethylcyclohexylethynyl and 2-fluoro-4-bromoiodobenzene, 1-[(anti)-4-ethylcyclohexylethynyl]-2- Fluoro-4-bromobenzene.
[0019] In a 500ml four-necked flask, add 200ml triethylamine, 13.6g (0.1mol) (trans)-4-ethylcyclohexylacetylene, 46.5g (0.15mol) 1-bromo-2-fluoroiodobenzene, 0.4g tetra( Triphenylphosphine) palladium dihalide, 0.4g cuprous iodide, 0.8g triphenylphosphine, stirred and refluxed for 12 hours, cooled to room temperature, added 80ml saturated ammonium chloride solution and 80ml dichloroethane, stirred for several minutes, Separate the organic phase, extract the aqueous phase with 3 x 60ml of dichloromethane, combine the organic phases, wash with 3 x 200ml of water until neutral, dry over anhydrous sodium sulfate, spin off the solvent, and use petroleum ether (60-90) for the residue Rec...
Embodiment 2
[0067] Example 2: Preparation of liquid crystal monomer 1,4-bis[(trans)-4-ethylcyclohexylethynyl]-2-fluorobenzene
[0068] From (anti)-4-ethylcyclohexylacetylene and 2,5-bromofluorobenzene, 1,4-bis[(anti)-4-ethylcyclohexylethynyl]-2-fluorobenzene was prepared by Heck reaction liquid crystal monomer.
[0069] In a 500ml four-necked flask, add 200ml triethylamine, 29.8g (0.1mol) (trans)-4-ethylcyclohexylacetylene, 14.0g, 0.45g 2-fluoro-1,4-dibromobenzene, 0.4g tetrakis( Triphenylphosphine) palladium dihalide, 0.4g cuprous iodide, 0.8g triphenylphosphine, stirred and refluxed for 12 hours, cooled to room temperature, added 80ml saturated ammonium chloride solution and 80ml dichloroethane, stirred for several minutes, Separate the organic phase, extract the aqueous phase with 3 x 60ml of dichloromethane, combine the organic phases, wash with 3 x 200ml of water until neutral, dry over anhydrous sodium sulfate, spin off the solvent, and use petroleum ether (60-90) for the residue ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com