Glycosyl isoxazole compounds and their preparation methods and fungicides
A compound, isoxazole technology, applied in the preparation of sugar derivatives, botanical equipment and methods, fungicides, etc., can solve the problems of large agricultural production losses, fungicides resistance, etc., achieve excellent inhibitory activity, obvious mycelium Effect of growth inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] The present invention also provides a preparation method for glycosylisoxazole compounds, comprising the following steps:
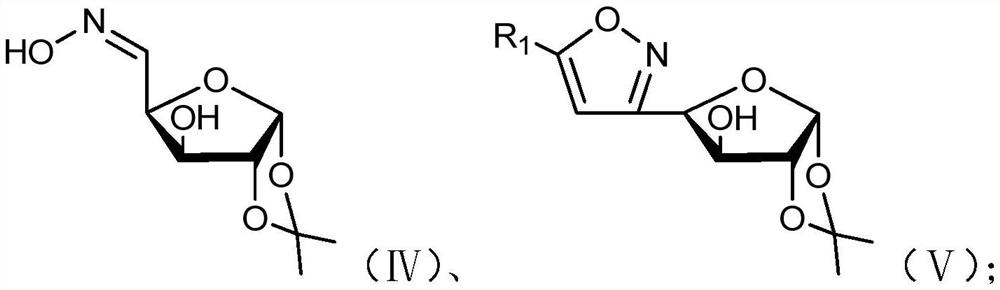
[0075] (a) When R 2 When it is H, compound IV and Under the effect of oxidizing agent and catalyst, react to obtain compound V;
[0076] Wherein, the structural formulas of compound IV and compound V are respectively as follows:
[0077]
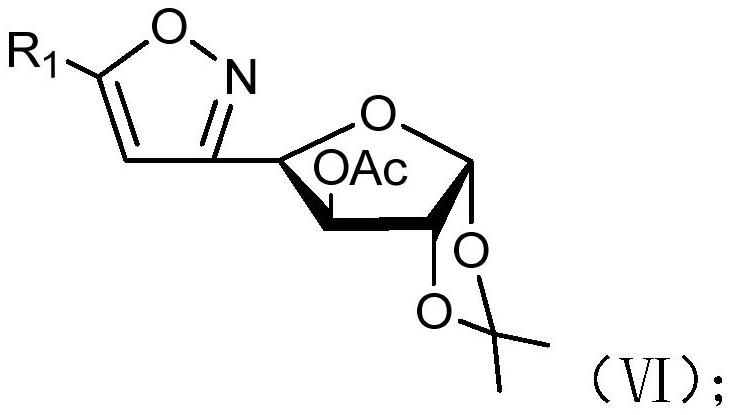
[0078] (b) when R 2 When it is an acetyl group, compound V reacts with an acetylating reagent under the action of an organic base to obtain compound VI;
[0079] Wherein, the structural formula of compound VI is as follows:
[0080]
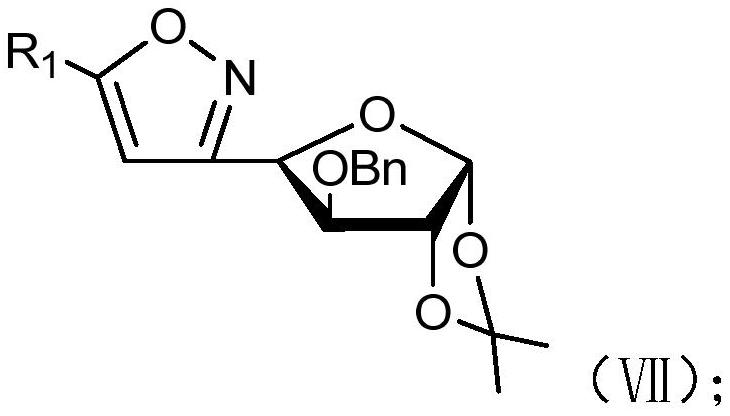
[0081] (c) When R 2 When it is benzyl, compound V reacts with a benzylation reagent under the action of sodium hydride to obtain compound VII;
[0082] Wherein, the structural formula of compound VII is as follows:
[0083]
[0084] (d) when R 2 When it is a propargyl group, compound V reacts with a propargylating reagent under the action of sodium hydride to obtai...
Embodiment 1
[0123] This embodiment provides glycosyl isoxazole compound V 3 And preparation method thereof, synthetic route is as follows:
[0124]
[0125] The specific synthesis steps are as follows:
[0126] Step (i): Weigh 20g of pure diacetone dextrose and pour it into a 1000mL dry round bottom flask, pressurize and pump 400mL of anhydrous pyridine, stir well to dissolve. Slowly add 13.07 mL of acetic anhydride to the reaction system dropwise in an ice-water bath and keep the temperature not exceeding 15°C, remove the ice-water bath, stir at room temperature for 2 hours, and detect by TLC [V (petroleum ether): V (ethyl acetate) = 3: 1] The response is complete. Post-treatment: filter the reacted material with suction and collect the filtrate, concentrate, add dichloromethane to dissolve the concentrated product and extract (3 times, 300 mL each), wash with deionized water (2 times, 150 mL each), and combine the organic phases Then add anhydrous sodium sulfate to dry, filter wit...
Embodiment 2
[0132] This embodiment provides glycosyl isoxazole compound V 1 For its preparation method, refer to the synthetic route of Example 1, the only difference is that the p-chlorophenylacetylene in step (v) is replaced by equimolar phenylacetylene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com