Aromatic amine derivative containing benzophenone group and preparation method and application thereof
A technology of aromatic amines and benzophenone, which is applied in the field of aromatic amine derivatives and their preparation, can solve the problems of low luminous intensity, efficiency roll-off, and low external quantum efficiency of phosphorescent OLEDs, etc., and achieve good hole transport ability, low cost, and the effect of improving external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
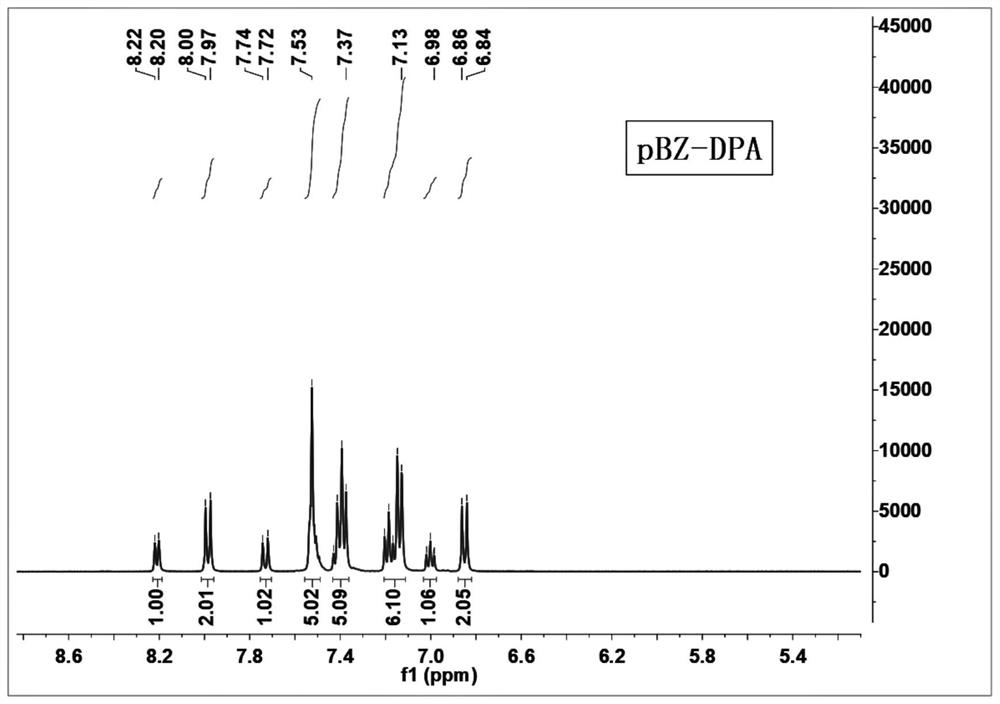
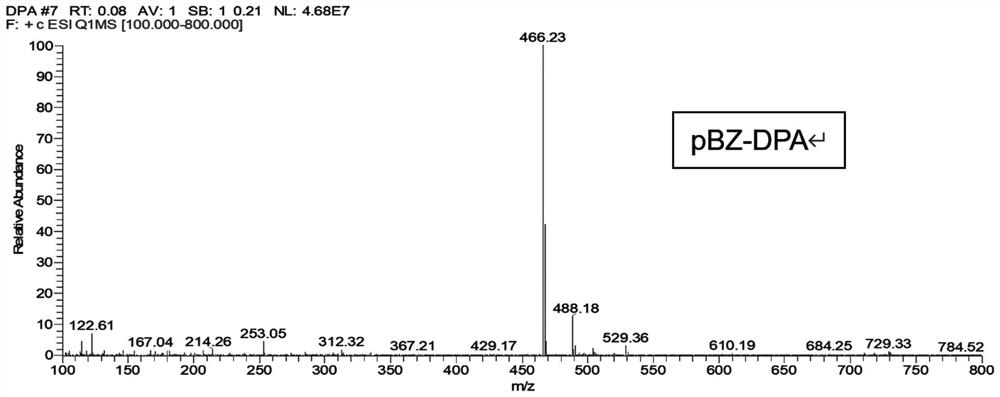
[0060] An aromatic amine derivative containing a benzophenone group has a molecular structure as shown in formula (I), named pBZ-DPA:
[0061]
[0062] The preparation method of the above-mentioned aromatic amine derivatives containing benzophenone group comprises the following steps:
[0063] S1. Preparation of (4-bromophenyl)(3-phenylimidazo[1,2-a]pyridin-2-yl)methanone:
[0064] Weigh (E)-1-(4-bromophenyl)-3-phenylprop-2-en-1-one 2.87g, 2-aminopyridine 0.94g, iodine 2.53g, 0.154g and 20mL chloroform in In a two-necked flask, a Michael ring closure reaction was carried out at a temperature of 75°C to obtain (4-bromophenyl)(3-phenylimidazo[1,2-a]pyridin-2-yl)methanone after treatment;
[0065] Its reaction equation is:
[0066]
[0067] S2. the preparation of formula (I) compound:
[0068] Weigh (4-bromophenyl)(3-phenylimidazo[1,2-a]pyridin-2-yl)methanone 188mg, diphenylamine 101mg, sodium tert-butoxide 72mg, tri-tert-butylphosphine 30mg, [1,3-bis(2,6-diisopropylphe...
Embodiment 2
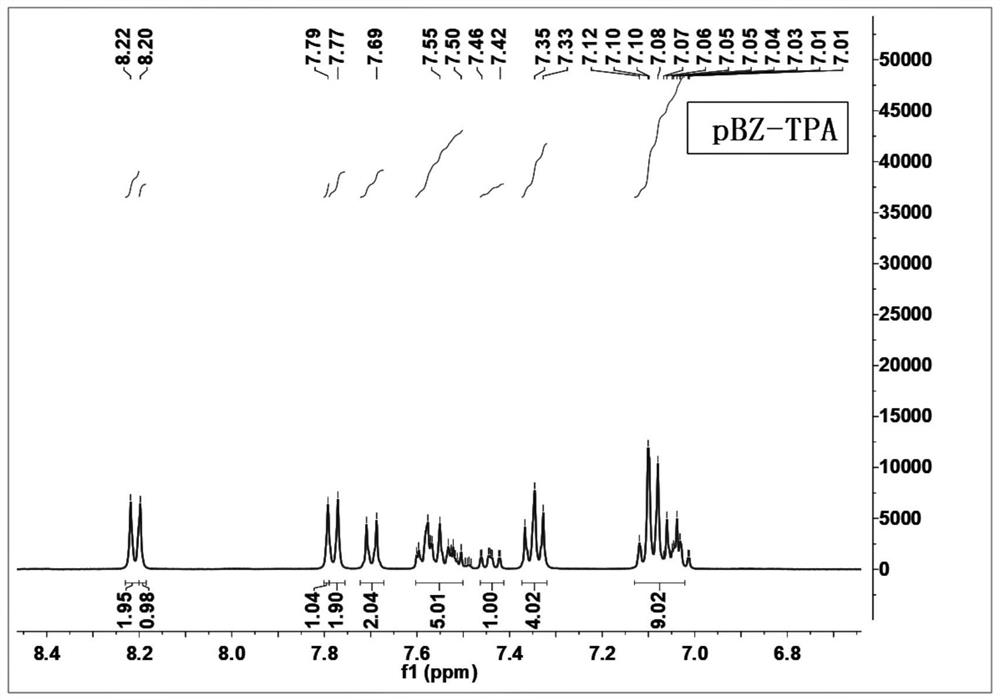
[0072] An aromatic amine derivative containing a benzophenone group has a molecular structure as shown in formula (II), named pBZ-TPA:
[0073]
[0074] The preparation method of the above-mentioned aromatic amine derivatives containing benzophenone group comprises the following steps:
[0075] S1. The preparation of (4-bromophenyl)(3-phenylimidazo[1,2-a]pyridin-2-yl)methanone is the same as in Example 1;
[0076] S2. the preparation of formula (Ⅱ) compound:
[0077] Weigh (4-bromophenyl)(3-phenylimidazo[1,2-a]pyridin-2-yl)methanone 188mg, 4-triphenylamine borate 235mg, 2mol / L sodium carbonate aqueous solution 3ml, four (Triphenylphosphine)palladium 40mg and 5mL of toluene were placed in a reaction tube, heated, stirred and refluxed for 12 hours under the protection of nitrogen at 120°C. After the reaction, the crude product was cooled, distilled, extracted, dried, concentrated, and separated; collected by cooling A yellow turbid liquid was obtained, the turbid liquid was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com