Method for detecting related substances of vincamine acid and apovincamine acid in injection
A kind of technology of apo-vincine and vincine, which is applied in the field of detection of the related substances of vincine and apo-vincine in injection, can solve the problem of increasing the related impurities of synthetic starting materials and synthetic intermediates, which cannot be solved. Separation and other problems, to achieve the effect of ensuring stable quality, uniformity, curative effect, strong specificity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
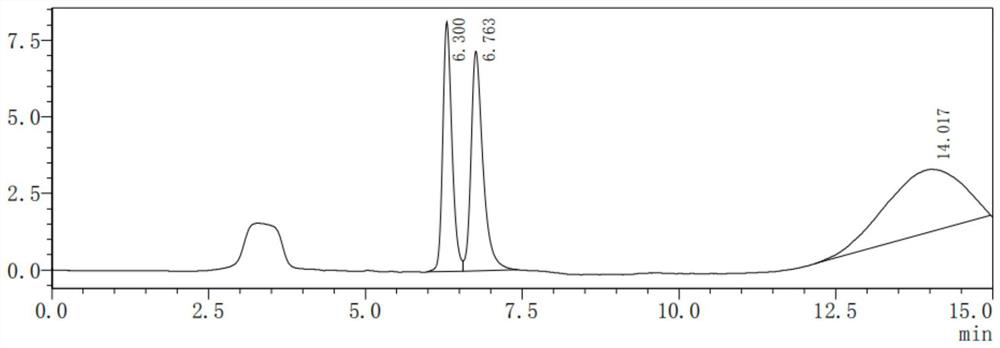
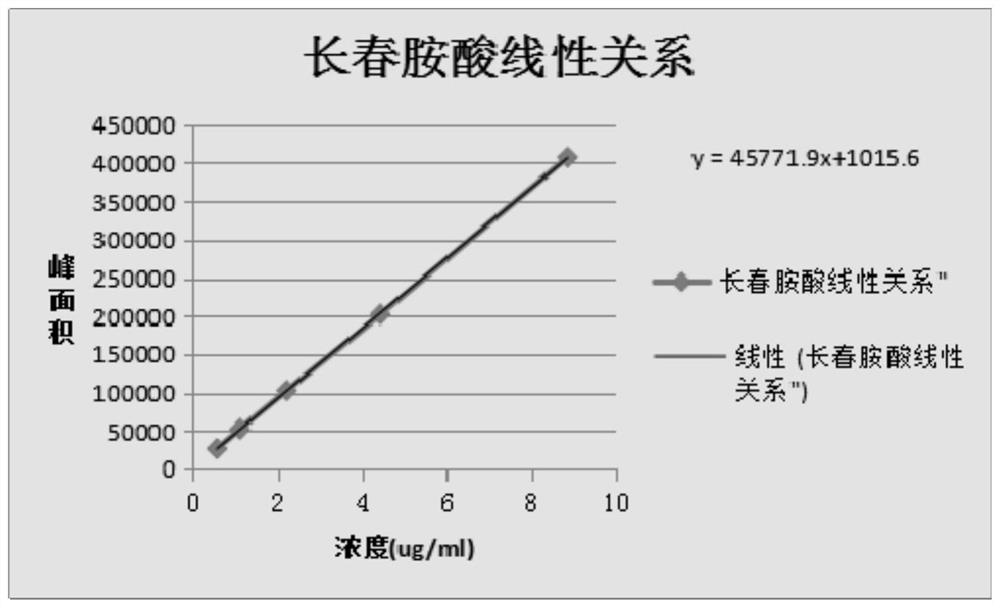
[0034] (1) Determination of the content of impurity vincine and impurity apovincine
[0035] (1) Precisely measure an appropriate amount of Vinpocetine injection, make a solution containing about 1.0 mg of Vinpocetine in every 1ml with mobile phase quantitative dilution, as the test solution;
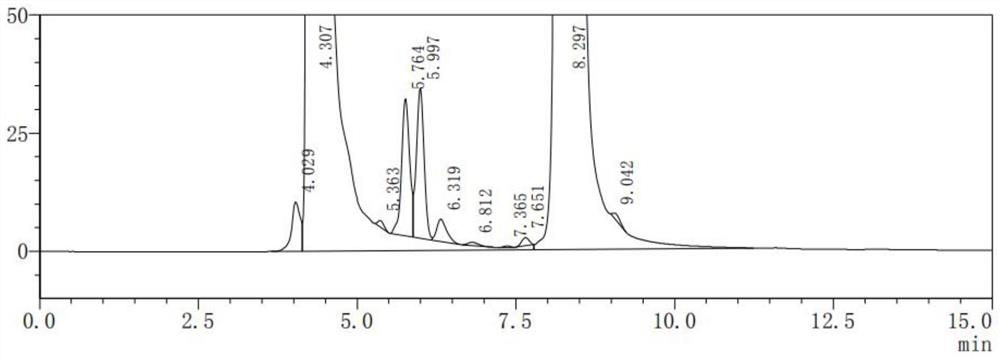
[0036] (2) Get the appropriate amount of impurity vincine and impurity apvincine reference substance, accurately weighed, add mobile phase to dissolve and quantitatively dilute to make each 1ml containing approximately impurity vincine, impurity apvincine 2 μg of mixed solution, as impurity reference substance solution;
[0037] (3) Get Vinpocetine reference substance 10mg, put in 10ml measuring bottle, dissolve and dilute to scale with impurity reference substance solution, shake up, as system suitability test solution;
[0038] (4) Take 20 μl of the system suitability test solution, inject it into the liquid chromatograph, and record the chromatogram. The order of the peaks is impuri...
Embodiment 2
[0050] Example 2 - The difference between this example and Example 1 is that a 0.25 mol / L triethylamine solution and ethanol with a volume ratio of 30:70 are used as the mobile phase. The results showed that the peak shapes of vinpocetine, vinblastine and apovincine were good.
Embodiment 3
[0051] Example 3 - The difference between this example and Example 1 is that 0.1 mol / L triethylamine solution and ethanol with a volume ratio of 1:1 are used as the mobile phase. The results showed that the peak shapes of vinpocetine, vinblastine and apovincine were good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com