Synthetic method of non-steroidal antiinflammatory drug pain killing
A technology for a non-steroidal anti-inflammatory drug and a synthetic method, applied in the field of drug synthesis, can solve the problems of poor commercialization, unsuitable storage, high price, etc., and achieve the effects of reducing process cost, cheap process raw materials, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
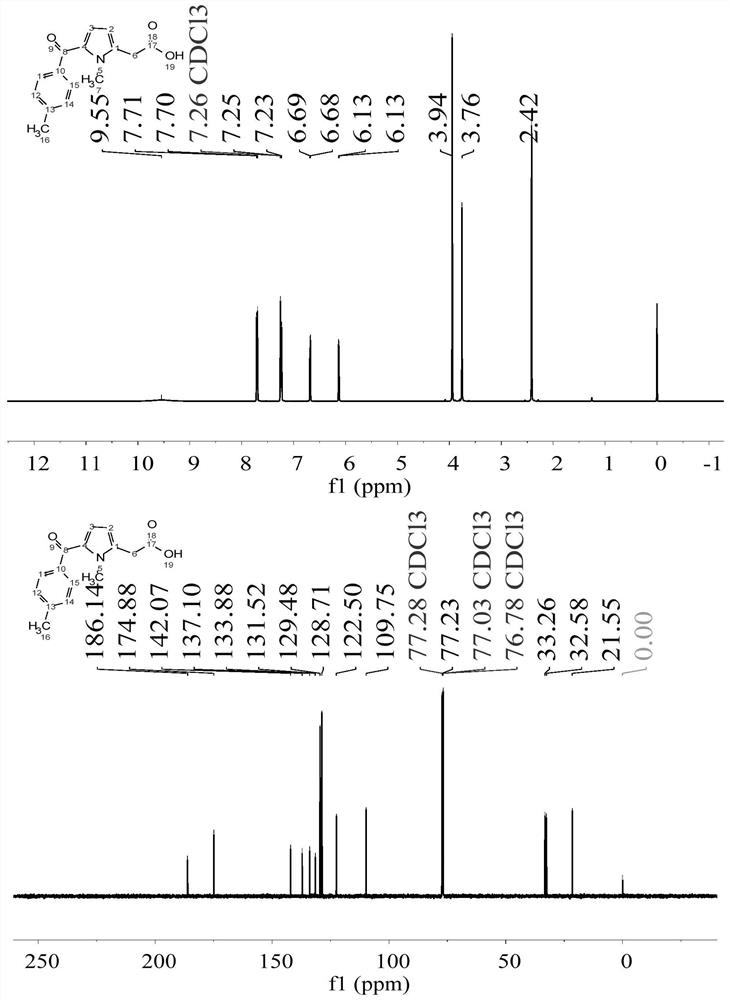
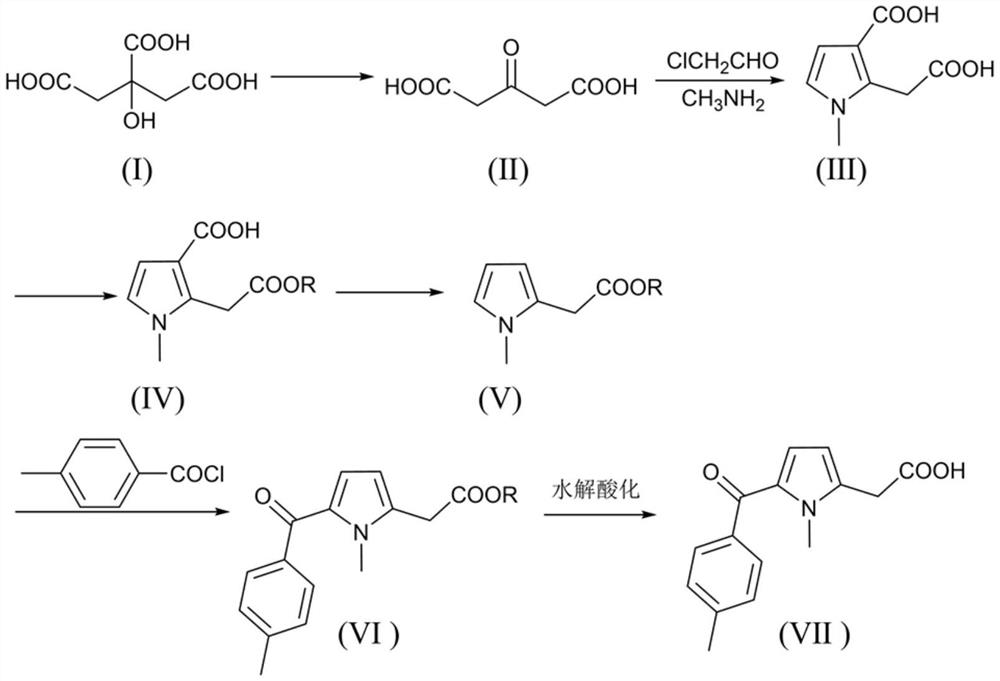
Image
Examples
Embodiment 1
[0042] Synthesis of Acetone Dicarboxylic Acid
[0043]Add 1000g of concentrated sulfuric acid and 192g of citric acid into a 2000ml three-neck flask, stir and react at 50°C for 15h, add the reaction liquid to 1000ml of ice water for cooling and crystallization, and filter at low temperature to obtain 161.2g of crude acetone dicarboxylic acid containing sulfuric acid. Liquid chromatography detected that the crude product contained 123.5 g of acetone dicarboxylic acid, and the yield was 84.5%.
[0044] Synthesis of 2-(2-acetoxy)-1-methyl-1H-pyrrole-3-carboxylic acid
[0045] 95.4g crude acetone dicarboxylic acid containing sulfuric acid (containing 73.1g acetone dicarboxylic acid), 60.0g 26% methylamine aqueous solution and 100g 40% chloroacetaldehyde aqueous solution were mixed, and stirred and reacted for 12h under an ice-water bath, and the reaction Afterwards, unreacted methylamine and chloroacetaldehyde were distilled off under reduced pressure, the temperature of the solu...
Embodiment 2
[0056] Synthesis of Acetone Dicarboxylic Acid
[0057] Add 2000g of concentrated sulfuric acid and 192g of citric acid into a 2000ml three-neck flask, stir and react at 10°C for 15h, add the reaction liquid to 1000ml of ice water for cooling and crystallization, and filter at low temperature to obtain 103.5g of crude acetone dicarboxylic acid containing sulfuric acid. Liquid chromatography detected that the crude product contained 77.9 g of acetone dicarboxylic acid, and the yield was 53.3%.
[0058] Synthesis of 2-(2-acetoxy)-1-methyl-1H-pyrrole-3-carboxylic acid
[0059] Mix 97.0 g of crude acetone dicarboxylic acid containing sulfuric acid (containing 73.0 g of acetone dicarboxylic acid), 360.0 g of 40% aqueous solution of methylamine and 200.0 g of 40% aqueous solution of chloroacetaldehyde, and stir the reaction in an ice-water bath for 12 h, After the reaction, the unreacted methylamine and chloroacetaldehyde were distilled off under reduced pressure, the temperature of...
Embodiment 3
[0069] Synthesis of Acetone Dicarboxylic Acid
[0070] Add 500g of concentrated sulfuric acid and 192g of citric acid into a 2000ml three-necked flask, stir and react at 70°C for 15h, add the reaction liquid to 1000ml of ice water for cooling and crystallization, and filter at low temperature to obtain 142.8g of crude acetone dicarboxylic acid containing sulfuric acid. Liquid chromatography detected that the crude product contained 111.4 g of acetone dicarboxylic acid, and the yield was 76.2%.
[0071] Synthesis of 2-(2-acetoxy)-1-methyl-1H-pyrrole-3-carboxylic acid
[0072] 93.8g of crude acetone dicarboxylic acid containing sulfuric acid (containing 73.2 g of acetone dicarboxylic acid), 770 g of 40% methylamine aqueous solution and 295.0 g of 40% chloroacetaldehyde aqueous solution were mixed, and stirred and reacted in an ice-water bath for 12 h, the reaction Afterwards, the unreacted methylamine and chloroacetaldehyde were distilled off under reduced pressure, the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com