HPLC-DAD based six-ingredient rehmannia pill prescription analysis and quality analysis method
A technology of Liuwei Dihuang Pills and a quality control method, which is applied in the field of quality control of the compound preparation Liuwei Dihuang Pills, which can solve the problems of cumbersome cost and methods, low TLC accuracy, and difficult DNA extraction and analysis operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Preparation of model Liuwei Dihuang Pills: Weigh 10g each of Rehmannia glutinosa, wine cornus, Moutan cortex, Chinese yam, Poria cocos and Alisma alisma, crush them and pass through No. 1.0047g of wine cornus powder, 0.7528g of Moutan cortex powder, 1.0009g of Chinese yam powder, 0.7508g of Poria cocos powder, 0.7520g of Alisma powder, and the model Liuwei Dihuang Wan was prepared according to the regulations of "Chinese Pharmacopoeia" 2015 edition. The above decoction pieces Rehmannia glutinosa are produced in Henan, wine cornus is produced in Henan, peony bark is produced in Anhui, yam is produced in Henan, Poria cocos is produced in Anhui, Alisma is produced in Fujian;
[0048] (2) Extraction of single-taste decoction pieces: Weigh 1.0001g of Rehmannia glutinosa powder, 1.0004g of wine cornus powder, 1.0034g of Moutan bark powder, 1.0006g of Chinese yam powder, 1.0029g of Poria cocos powder, and 1.0032g of Alisma powder, respectively. , add 50mL of 50% methanol, ...
Embodiment 2
[0056] Embodiment 2: Except for the quality evaluation process of commercial Liuwei Dihuang Pills, other steps are the same as in Embodiment 1.
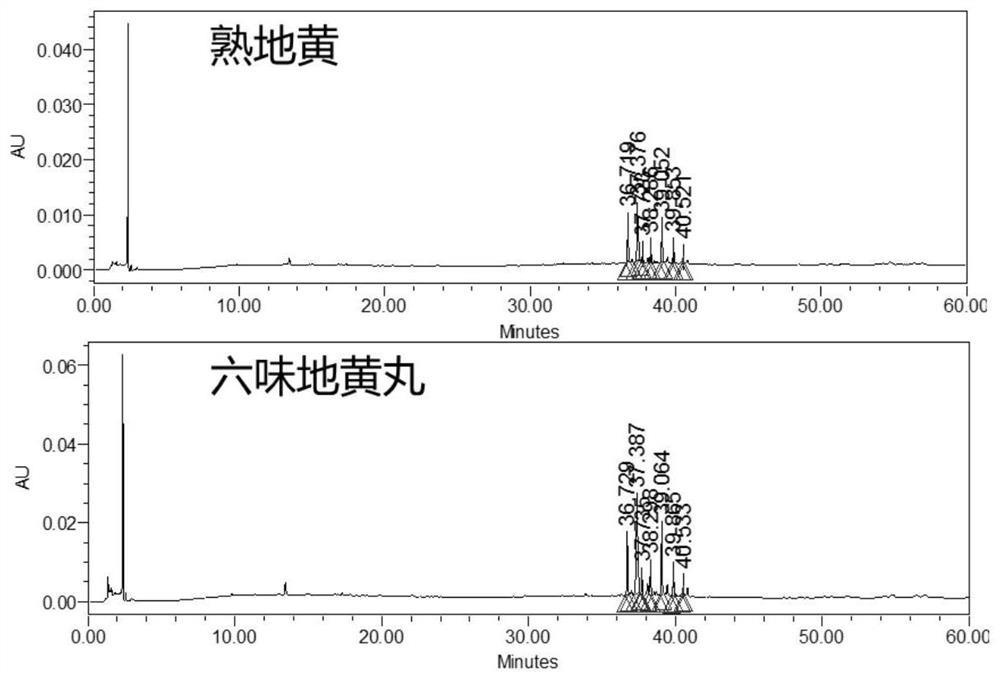
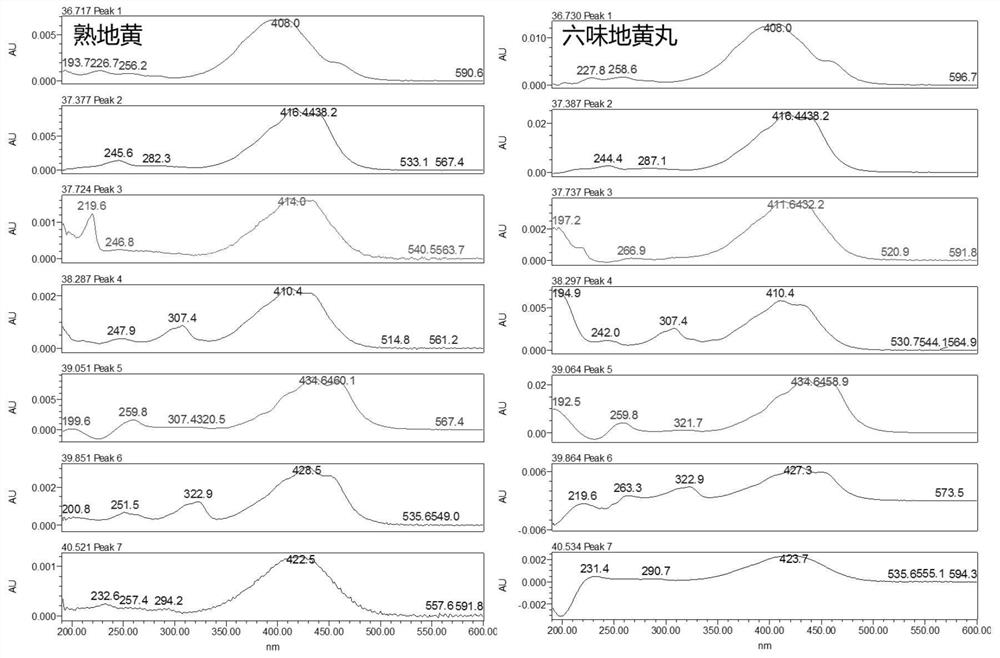
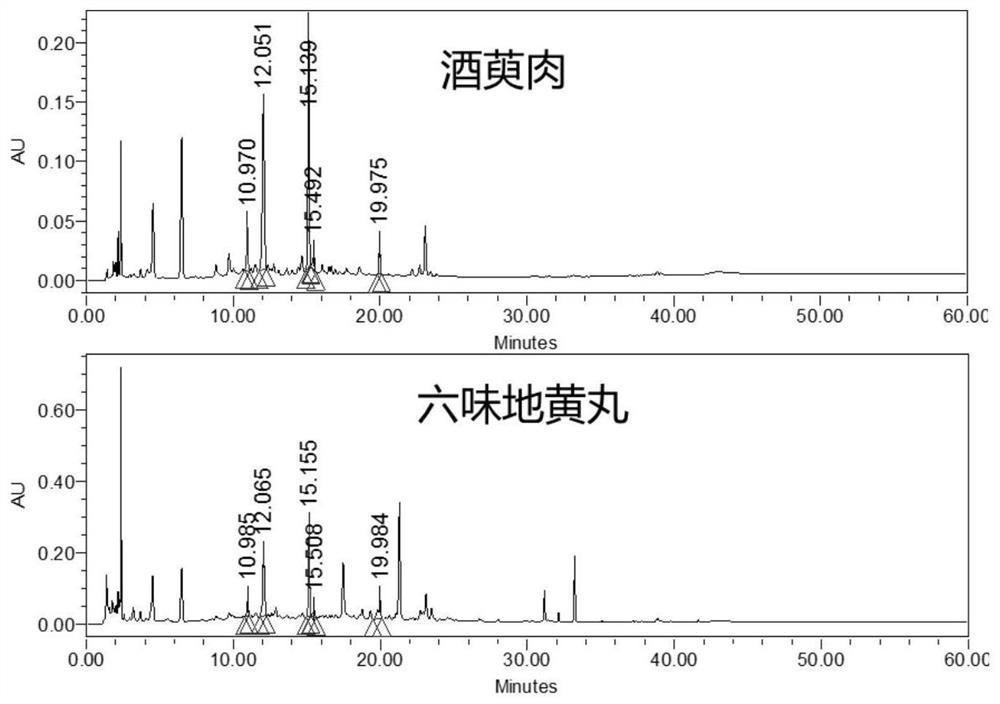
[0057] In the quality evaluation process of Erliuwei Dihuang Pills from the manufacturer, Q-Markers different from those in Example 2 were used: No. 4 characteristic peak of Rehmannia glutinosa, No. 2 characteristic peak of wine cornel, No. The No. 3 characteristic peak of Chinese yam, the No. 2 characteristic peak of Poria cocos, and the No. 2 characteristic peak of Alisma are Q-Markers for quality evaluation of the manufacturer Erliuwei Dihuang Pills. The evaluation results are shown in the attached Figure 20 .
Embodiment 3
[0058] Embodiment 3: Except for the extraction process of decoction pieces and Liuwei Dihuang Wan, other steps are the same as in Embodiment 1. The extraction process of single-flavor decoction pieces is to weigh 4.0012g of crushed Rehmannia glutinosa, 4.0005g of wine cornus, 4.0002g of Moutan cortex, 4.0017g of Chinese yam, 4.0025g of Poria cocos, 4.0014g of Alisma, add 200mL of 50% methanol, and heat to reflux Centrifuge after extraction for 60 min, and take the supernatant to pass through a 0.45 μm organic filter membrane for analysis.
[0059]The extraction process of Liuwei Dihuang Wan is to weigh the model Liuwei Dihuang Wan 4.0004g, manufacturer Yiliuwei Dihuang Wan 8.2016g, manufacturer Erliuwei Dihuang Wan 6.0007g, manufacturer Sanliuwei Dihuang Wan 6.0007g, manufacturer Siliuwei Dihuang Wan 8.2010 g, 6.0014g of Wuliuwei Dihuang Pills from the manufacturer, 8.2012g of Liuliuwei Dihuang Pills from the manufacturer, add 200mL of 50% methanol, heat and reflux for extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com