Treatment of chronic cough, breathlessness and dyspnea
A technology for shortness of breath and dyspnea, applied to respiratory diseases, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of cough medicine treatment, effective treatment, etc., to reduce hospitalization rate, lung fibrosis The effect of reducing the progress rate of chemical transformation and reducing fatigue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0256] 30 mg, 60 mg or 180 mg extended release (ER) nalbuphine tablets were prepared as follows: Add nalbuphine hydrochloride, mannitol, xanthan gum, locust bean gum and calcium sulfate dihydrate to a high shear mixer and Dry the mixture. The granulation solution (water for injection or purified water) was introduced into the mixer at low speed. Wet granulation is granulation at high speed and drying in a fluid bed processor. The dried granules are milled and classified using a conventional mill. Transfer the milled granules to a diffusion (drum) mixer. Hydroxypropylcellulose and (where applicable) fumaric acid (180 mg formulation only) were added to the diffusion mixer and blended. Afterwards, the magnesium stearate was added to the diffusion mixer and blended. The final blend was compressed using a rotary tablet press. Tablets may be coated with a non-functional Opadry white coating.
[0257] Table 1
[0258] 30mg, 60mg, 120mg and 180mg extended release nalbuphine tab...
Embodiment 2
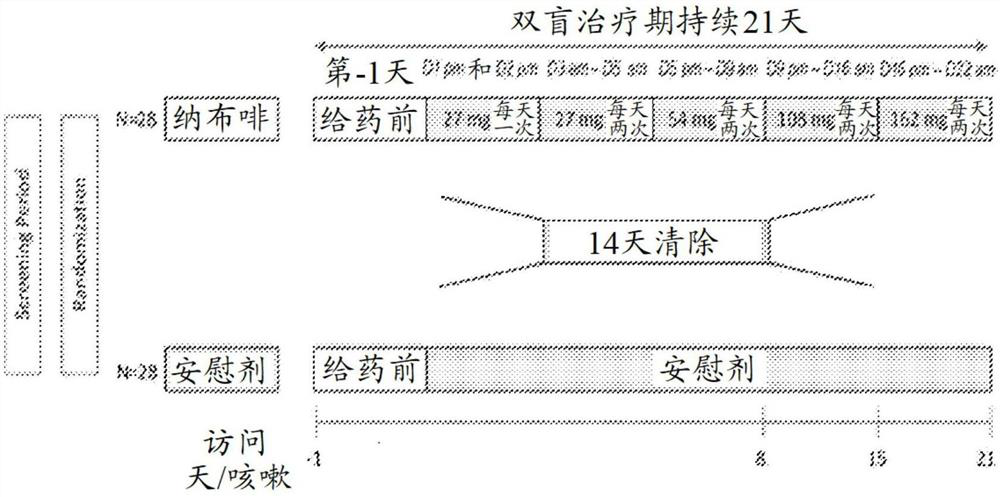
[0271] A double-blind, randomized, placebo-controlled, 2-cycle cross-over safety with nalbuphine hydrochloride ER tablets will be conducted in subjects with idiopathic pulmonary fibrosis for the treatment of cough, shortness of breath and dyspnea according to the following protocol: Sexuality and Efficacy Studies.
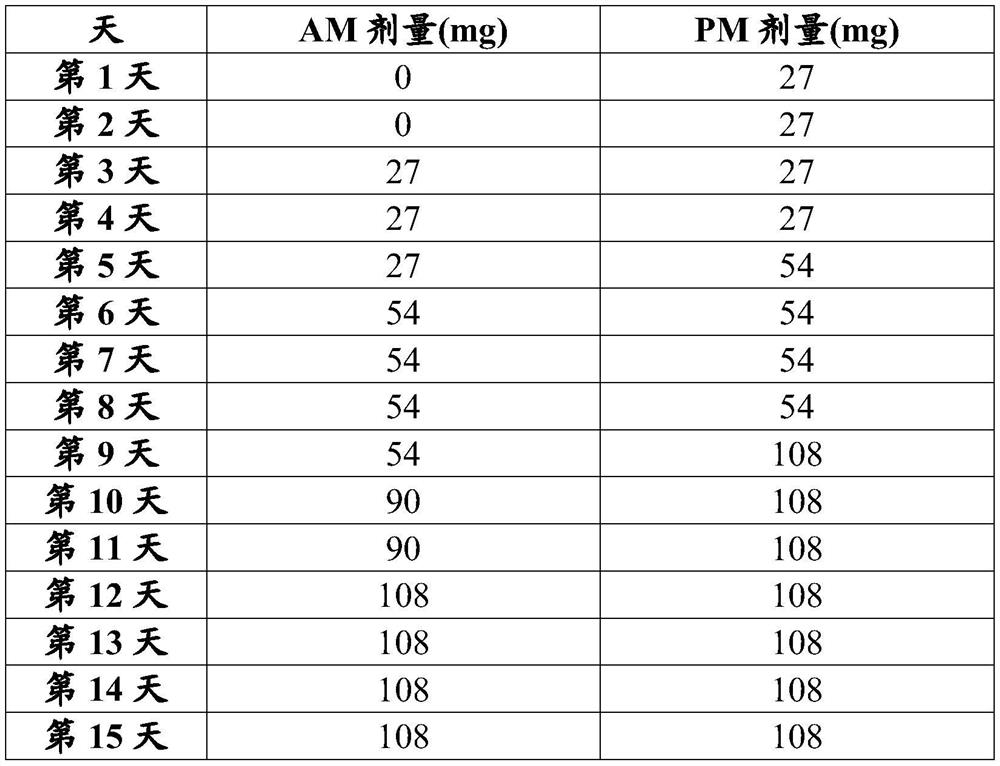
[0272] The study included two 3-week treatment periods separated by a 2-week washout period ( figure 1 ). During the first treatment period, patients were randomized 1:1 to a target dose of 162 mg twice daily (BID) of nalbuphine ER tablets (the equivalent dose of nalbuphine free base) or placebo tablets BID.
[0273] The primary objectives were to evaluate the effect of nalbuphine hydrochloride ER tablets on (1) percent change in daytime cough frequency and (2) as well as safety and tolerability. Daytime was defined as the time period during which subjects were awake within 24 hours of application of the digital cough monitor. Evaluation was performed using obj...
Embodiment approach
[0373] 1. A method of treating idiopathic pulmonary fibrosis (IPF) cough, shortness of breath or dyspnea comprising administering an effective amount of nalbuphine or a pharmaceutically acceptable salt thereof to a patient in need of such treatment or ester.
[0374] 2. The method of embodiment 1, wherein prior to said treatment, said patient has a daytime cough severity of at least 4 according to a Numerical Rating Scale for Cough Severity.
[0375] 3. The method of embodiment 1, wherein prior to said treatment, said patient's average daytime cough count is at least 15 cough counts per hour as measured using a cough count monitoring device.
[0376] 4. The method of embodiment 1, wherein the IPF cough is a chronic cough.
[0377] 5. The method of embodiment 1, wherein the IPF cough is refractory chronic cough.
[0378] 6. The method of embodiment 1, wherein the IPF cough is refractory to treatment with an antitussive selected from the group consisting of gefapiride, serlopi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com