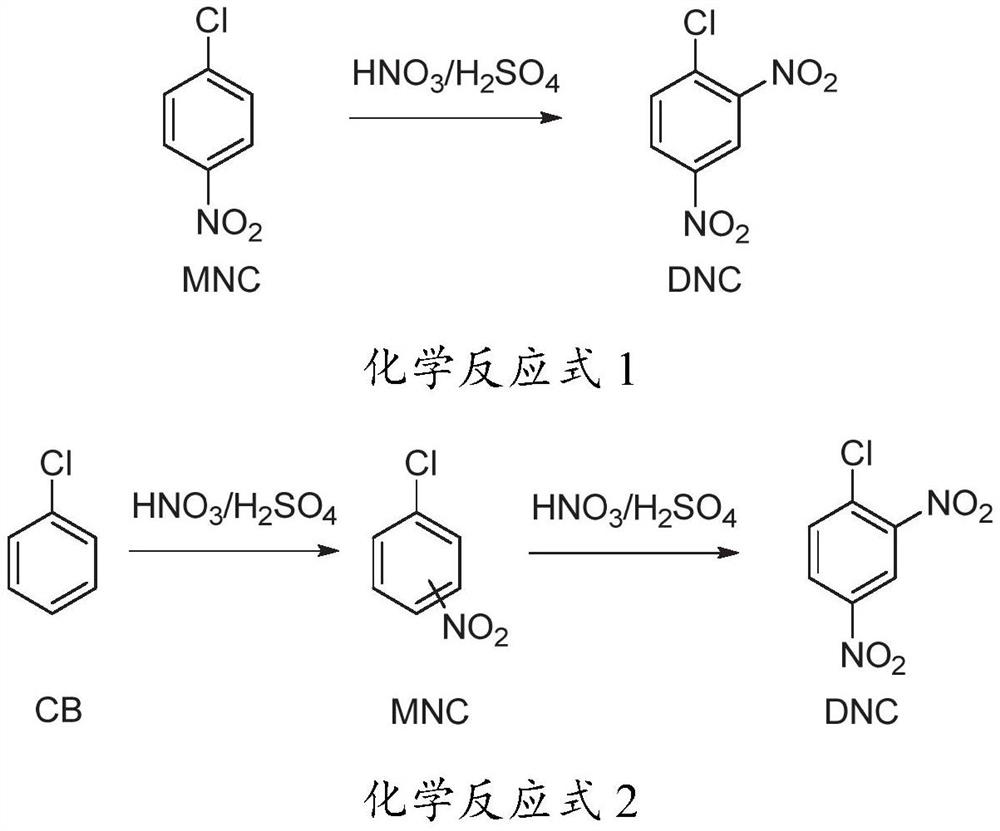
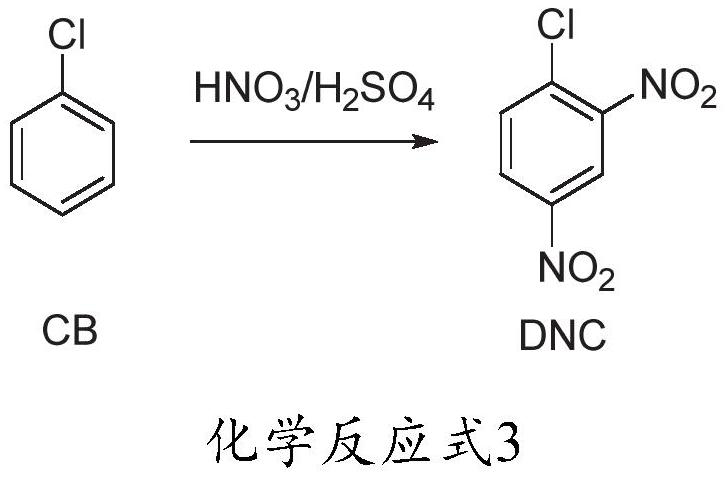
Method for preparing 2, 4-dinitrochlorobenzene by one-step adiabatic continuous nitration of chlorobenzene
A one-step technology of dinitrochlorobenzene and chlorobenzene, which can be used in the preparation of nitro compounds, chemical instruments and methods, organic chemistry, etc., and can solve problems such as energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
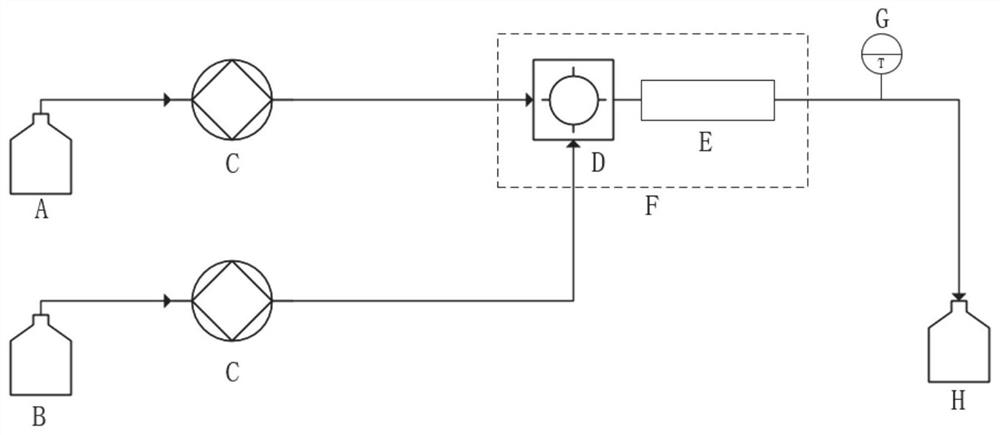
[0052] (1) 98% sulfuric acid and 98% nitric acid are prepared into a sulfuric acid solution of nitric acid in a mass ratio of 12:1, and stored in a mixed acid storage bottle A.
[0053] (2) chlorobenzene and 2,4-dinitrochlorobenzene are prepared into oil phase solution by mass ratio of 1.5:1, and stored in oil phase storage bottle B.
[0054] (3) The sulfuric acid solution of nitric acid and chlorobenzene (according to the molar ratio of nitric acid and chlorobenzene: 2.02:1) are respectively transported to the microreactor D using a high-precision high-pressure plunger pump C, and the inlet temperature is 30 ° C, under adiabatic conditions The reaction was carried out under the following conditions, the reaction residence time was 70 seconds, and the temperature sensor detected the outlet temperature of 125°C.
[0055] (4) The reaction liquid after the reaction flows into the reaction liquid storage bottle H.
[0056] (5) After the reaction solution is cooled to 80°C, take o...
Embodiment 2
[0059] The operation process is the same as in Example 1, except that the molar ratio of nitric acid and chlorobenzene is changed to 2.04:1, and the gas chromatography detection result is: mononitrochlorobenzene (0.2%), 2,4-dinitrochlorobenzene (99.8%) .
Embodiment 3
[0061] The operation process is the same as in Example 1, except that the molar ratio of nitric acid and chlorobenzene is changed to be 2.06:1, and the gas chromatography detection result is: 2,4-dinitrochlorobenzene (99.7%), trinitrochlorobenzene (0.3%) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com